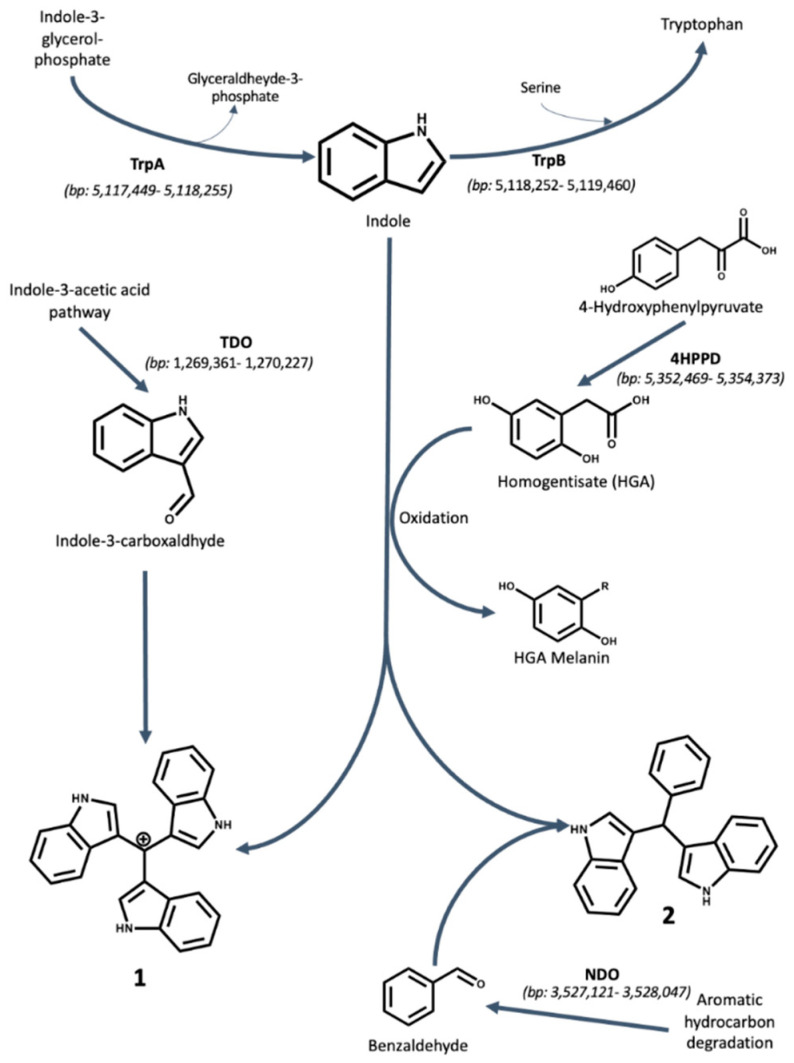
Figure 2.
Proposed biosynthesis mechanisms for Compounds 1 and 2 by P. aeruginosa UWI-1. Indole is proposed to originate from tryptophan degradation via an unclassified carbon–carbon lyase. The oxidative polymerization of homogentisate (HGA) to HGA melanin serves as the catalyst for the formation of compounds 1 and 2. Compound 1 is putatively formed via condensation of two molecules of indole and one molecule of indole-3-carboxaldehyde. Indole-3-carboxaldehyde is an intermediate product in the IAA synthesis pathway found in Pseudomonads. Compound 2 is putatively formed by the condensation of two molecules of indole and one molecule of benzaldehyde. Benzaldehyde is proposed to be derived as a product of polycyclic aromatic hydrocarbon metabolism by naphthalene 1,2-dioxygenase. The location of each gene involved in the biosynthesis is given in parentheses and is based on the genome of P. aeruginosa UWI-1 available from the European Nucleotide Archive (ENA) project number PRJEB32405 (https://www.ebi.ac.uk/ena/data/view/PRJEB32405).