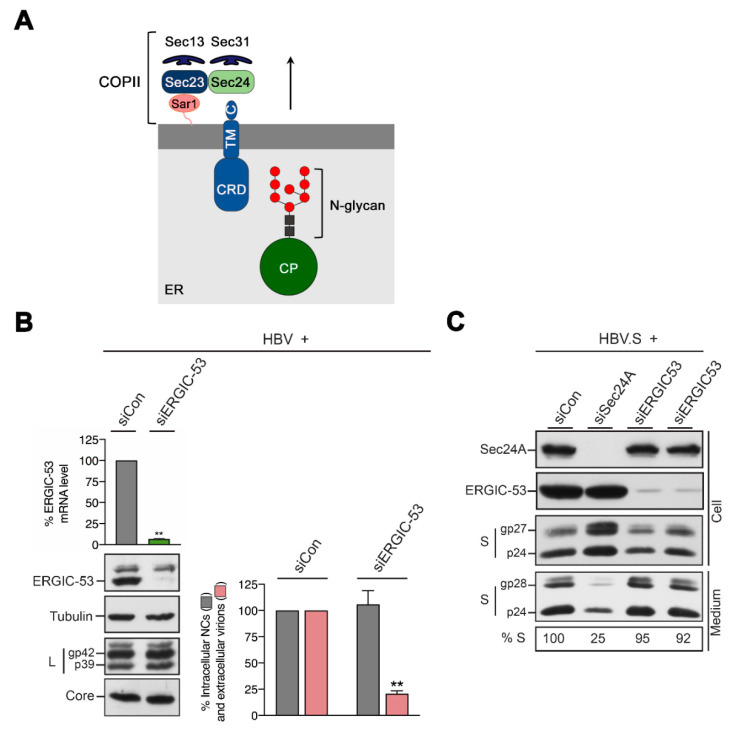
Figure 5.
ERGIC-53 controls the egress of HBV viral particles but not of HBV spherical subviral particles. (A) Model depicting the structure of ERGIC-53 in association with cargos and COPII. ERGIC-53 is a type I integral membrane protein with a C-terminal transmembrane domain (TM) and a luminal cargo recognition domain (CRD) interacting with N-linked high-mannose glycans of cargo proteins (CP) within the ER. Its cytoplasmic ER exit motif (C) binds to Sec24, the cargo adaptor of COPII. GTP activation of Sar1 leads to the formation of the Sec23/24 heterodimer, cargo capture and coat polymerization by the Sec13/31 complex. (B) The treatment of HuH-7 cells with the control (siCon) or ERGIC-53-specific siRNAs for 2 days and retransfection with the HBV* replicon construct for an additional 3 days. The degree of ERGIC-53 depletion was measured by qRT-PCR of the transcript levels and WB analysis. Cellular lysates and supernatants were subjected to tubulin-, L- and core-specific WB and to the virion production assay (n = 3, ± SD). (C) Treatment of HuH-7 cells with control (siCon), Sec24A- or ERGIC-53-specific siRNA duplexes for 3 days and retransfection with an haemagglutinin (HA)-tagged S construct (HBV.S) for an additional 2 days. The ERGIC-53-specific RNAi experiments were done in duplicate. To monitor depletion, cell lysates were examined by Sec24A- and ERGIC-53-specific WB. Cell lysates (Cell) and concentrated cell supernatants (Medium) were probed by anti-HA immunoblotting. The nonglycosylated (p24) and glycosylated (gp27 and gp28) forms of HBV.S are depicted.