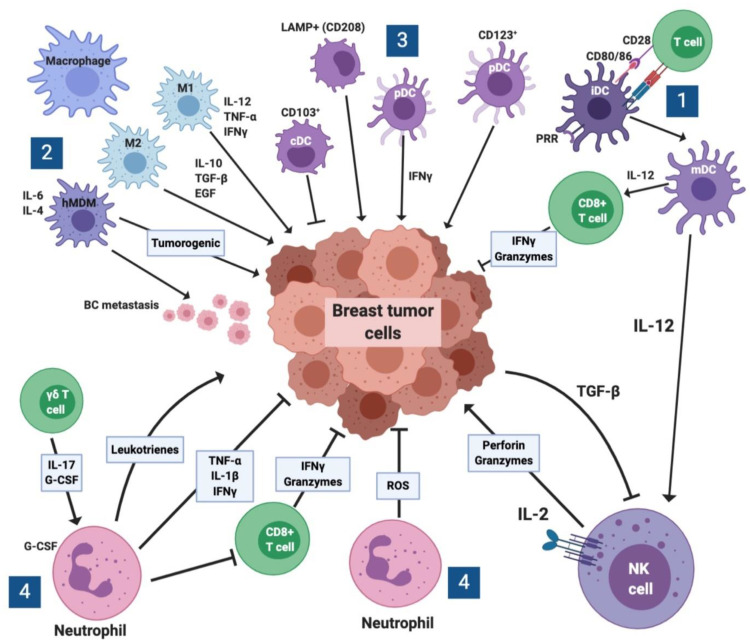
Figure 2.
Interplay among various cell types and inflammatory molecules in breast cancer microenvironment. The cells can be divided into subcategories. (1) Role of killer cells. Upon the cognate interaction among T cells and immature dendritic cells (iDCs), the latter which also express pattern recognition receptors (PRRs) differentiate into mature dendritic cells (mDCs). These cells secrete IL-12 which activates cytotoxic T cells to release granzyme B and IFN-γ, which kill breast cancer cells. IL-12 also activates NK cells which release perforin and granzyme B to lyse tumor cells. Reciprocally, breast cancer cells utilize TGF-β to inhibit the function of killer cells. (2) Role of macrophages. Human monocyte-derived macrophages (hMDM) facilitate tumorigenesis by releasing cytokines. Similarly, M2 macrophages potentiate breast cancer cells growth through the release of suppressive molecules such as IL-10, TGF-β, and VEGF, which suppress the cytolytic activity of anti-tumor effector cells. In contrast, macrophages type 1 (M1) suppress tumor growth through the release of inflammatory IL-12, TNF-α and IFN-γ. (3) Role of dendritic cells. CD208 or LAMP+ DCs as well as plasmacytoid dendritic cells (pDCs) enhance tumor growth, whereas conventional dendritic cells (cDCs) inhibit their growth. (4) Role of neutrophils. Neutrophils may exert opposing effects on breast cancer cells growth. On the one hand, it may secrete reactive oxygen species (ROS) that may affect the growth of breast cancer cells. However, neutrophils by receiving IL-17 and G-CSF from γδ T cells may secrete leukotrienes, which inhibit tumor growth, or secrete the inflammatory cytokines TNF-α, IL-1β, or IFN-γ that could suppress tumor growth. Finally, neutrophils also inhibit the cytolytic activity of CD8+ T cells.