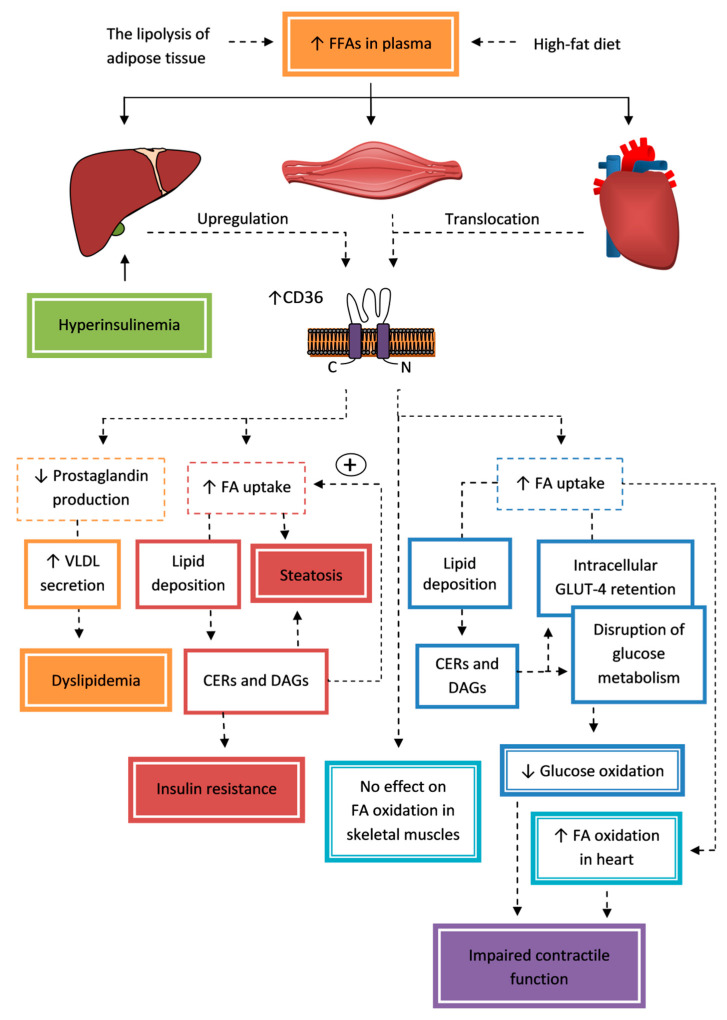
Figure 2.
The contribution of CD36 to the pathogenesis of insulin resistance in the liver, muscle, and the heart. Increased free fatty acid (FFA) concentrations in plasma are a factor initiating upregulation and translocation of CD36 with an increase in this transporter expression in the plasma membrane for the liver and myocytes. This leads to an increase in fatty acid (FA) influx via CD36 with pathological accumulation of triacylglycerols (TAGs), diacylglycerols (DAGs), and ceramides (CERs) initiating lipotoxicity and insulin resistance. A typical feature of an insulin-resistant liver is steatosis, in which accumulating lipids antagonize insulin signaling, re-increase CD36 expression in the cell membrane, disrupt mitochondrial function, and increase TAG accumulation. However, increased expression of CD36 in hepatocytes also stimulates the removal of FAs by very-low-density lipoprotein (VLDL) secretion, but this is associated with the worsening of dyslipidemia. Skeletal and cardiac myocyte overexposure to lipids results in glucose transporter type 4 (GLUT-4) being entrapped in non-endosomal storage and the translocation of CD36 to the sarcolemma associated with excessive FA transport and lipid accumulation interfering insulin signaling by a decreased glucose transport and its incorporation to glycogen. Moreover, in cardiomyocytes, FA oversupply changes the expression of genes associated with FA and glucose utilization. Ultimately, these actions result in a transition to energy from FAs instead of glucose and contractile dysfunction.