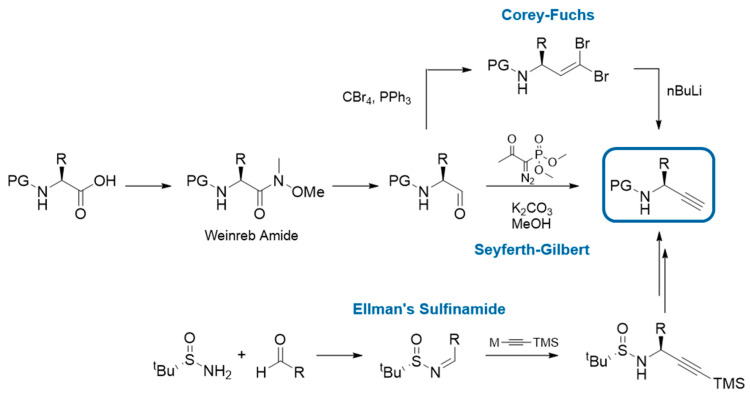
Figure 4.
Different approaches for the synthesis of α-amino alkynes: The most common synthesis is starting from an amino aldehyde via a Seyferth-Gilbert homologation using the Bestmann-Ohira reagent. Another possibility from the same starting material is the Corey-Fuchs reaction. An alternative approach is the condensation of an achiral aldehyde and Ellman’s auxilliary followed by the conjugate addition of an organometallic alkyne reagent to the sulfinamide. M = Metal (e.g., Li or MgX), PG = protecting group, R = amino acid side chain, TMS = trimethylsilyl.