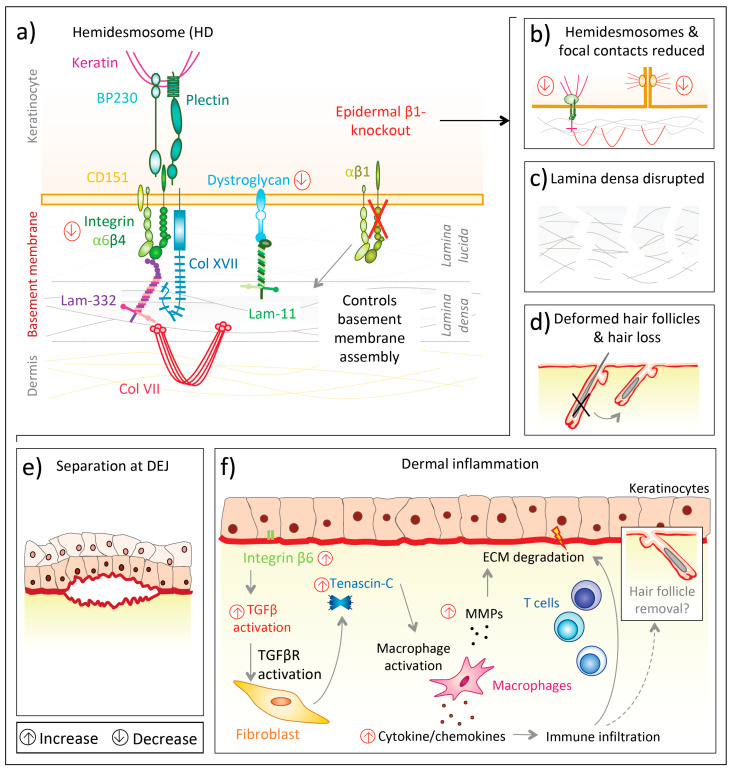
Figure 2.
Epidermal integrin β1 deficiency in mice and its consequences. (a) Loss of epidermal integrin β1 decreases the laminin receptor dystroglycan and integrin α6β4, which is a major component of hemidesmosomes [102,103]; (b) Integrin β1-deficient keratinocytes have fewer hemidesmosomes and focal adhesions [102]. The skin in epidermal integrin β1-deficient in mice presents with diminished lamina densa (c) [102], deformed hair follicles resulting in hair loss (d) [104] and (e) show separation at the dermal–epidermal junction (DEJ) and dermal inflammation (f) [105]. A proposed mechanism of the latter is that loss of integrin β1 increases epidermal integrin β6, which is involved in transforming growth factor β (TGFβ) activation. Increased TGFβ signaling upregulates, among others, tenascin-C, fostering dermal cytokine/chemokine production by macrophages, which leads to immune cell (T-cells, mast cells) recruitment. Macrophages also likely cause the increase of matrix metalloproteinases (MMPs) and all together extracellular matrix (ECM) degradation, modified from and inspired by Kurbet et al. [105].