Figure 4.
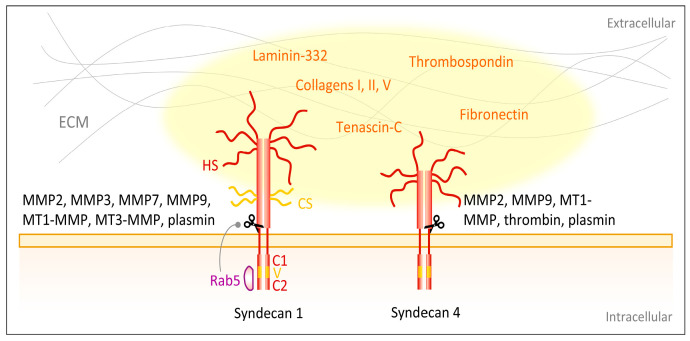
Syndecans interact with ECM components. Major skin syndecans are syndecan-1 and -4. They consist of extracellular signaling peptide containing glycosaminoglycans (GAGs), i.e., heparan sulfate (HS) and chondroitin sulfate (CS) chains, a transmembrane domain and intracellularly two conserved regions C1 and C2 separated by a variable (v) region [155,156,157,158]. Via the GAGs the syndecans interact with ECM ligands, such as laminin-332 [205,206], collagen I, III and V [214]. Additionally, proteins expressed in the transitional ECM of wounds, such as thrombospondin, fibronectin and tenascin-C are ligands [215,216,217,218]. Several proteinases shed the extracellular domain of syndecans, for example matrix metalloproteinases MMP2 [172,173], MMP3 [173], MMP7 [173], MMP-9 [172,173], MT1-MMP [173,174] and MT3-MMP [174] as well as the serine proteinases thrombin and plasmin [173]. The small GTPase Rab5 controls syndecan-1 shedding [176].