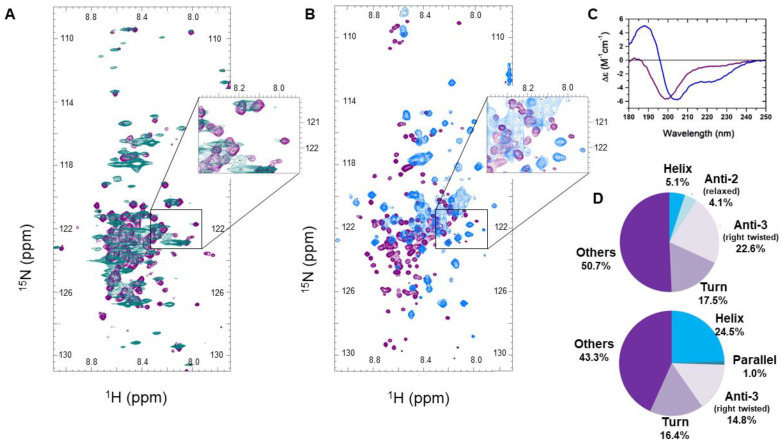
Figure 3.
Changes in the in-cell NMR spectrum of ERD14 correspond to intracellular interactions (A) In-cell 1H-15N HSQC spectrum of scrambled (Full-Scr) ERD14 (teal) is compared to the HSQC spectrum (purple) of purified Full-Scr ERD14 recorded in buffer. Broadenings of several resonances can be observed, but there is no peak disappearance in the region observed for WT ERD14 (insert, cf. also Figure 2A). For easier comparison, the zoomed region is the same as in case of WT ERD14; however, because of the scrambling, the exact sequences are not the same in the two proteins. An assignment of Full-Scr can be found in Supplementary Figure S1C (Supplementary Materials). (B) Comparison of the 1H-15N HSQC spectra of WT ERD14 measured in MES buffer (pH 6.5) (purple) and in the presence of 30% trifluoro-ethanol (TFE) (blue). As shown by circular dichroism (C,D) measurements (C), TFE at this concentration induces conformational changes in ERD14, yet it only causes peak shifts and broadening, no signal disappearances (Panel (B), insert). (D) Analysis of circular dichroism (CD) spectra by BeStSel [59] suggests that WT ERD14 is mostly disordered (a-helix content 5%), whereas in the presence of 30% TFE, it assumes a significant secondary structure (α-helix content is 24%).