Figure 4.
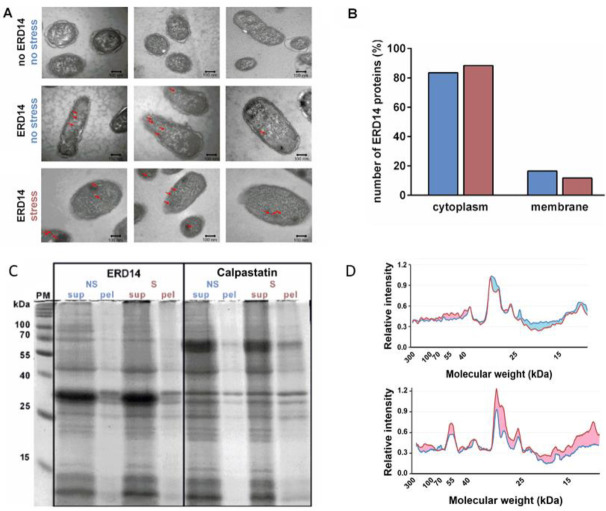
ERD14 protects the proteome of the cells. (A) ERD14 is primarily localized in the cytoplasm and does not translocate to the cell membrane upon stress, as shown by electron microscopy (EM). FLAG-tagged ERD14 was overexpressed in E. coli cells, which were fixed by formaldehyde either before (second line) or after (third line) heat stress (50 °C × 15 min). Slices of fixed cells were immunostained by anti-FLAG primary and nanogold-conjugated secondary antibody. Red arrows show gold particles corresponding to ERD14 protein, indicating a primarily cytoplasmic localization both before and after stress. No staining is seen in cells in which ERD14 was not overexpressed (first line). (B) Analysis of 100 gold particles show that 84% and 88% of the observed particles are localized in the cytoplasm before (blue) and after (purple) heat stress, respectively, whereas the low percentage of particles localized next to the membrane does not increase upon stress. (C) ERD14 protein has a general protective effect on the proteome, as shown by the pattern of proteins in solution (supernatant, sup) and pellet (pel) on 12.5% SDS-PAGE gels in cell extracts of cells overexpressing ERD14 or calpastatin, before (no stress, NS) and after (stress, S) heat stress. (D) Densitometric analysis of pellets before (blue line) and after (red line) stress was carried out using ImageJ image processing software. Differences are colored for better clarity. The results suggest that after heat stress, proteins within the whole Mw range get more aggregated in the presence of calpastatin but remain more in solution in the presence of ERD14 (the observed differences are significant).