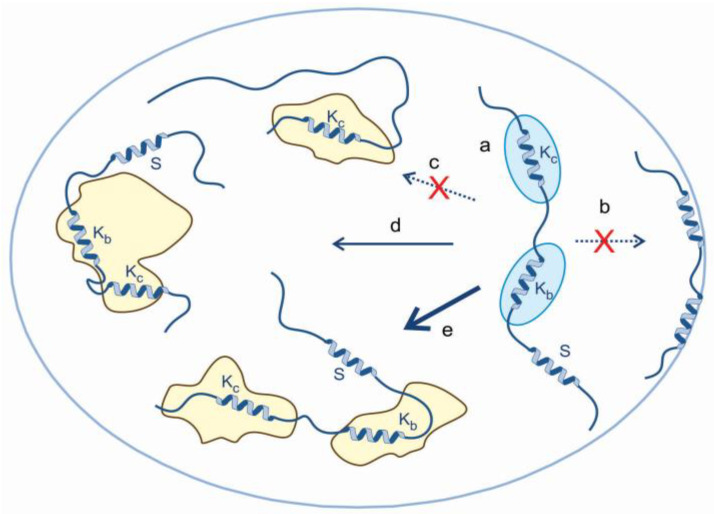
Figure 6.
A general mechanistic model of the chaperone activity of ERD14 in vivo. Our in vivo observations alongside previous in vitro data on the protection of ERD14 of the proteome can be rationalized by a model that combines the binding functions of short conserved motifs and space filling (entropic separation) by disordered linking and flanking regions. The model incorporates the following elements: (a) under non-stressed conditions, largely disordered ERD14 is loosely associated with native proteins, with its multivalent binding is mediated by its short binding regions (exemplified by Kb and Kc), while its S segment is unbound. Upon stress, many proteins in the cell become structurally compromised and expose hydrophobic regions; thus, ERD14 can engage with them, rather than the cell membrane (b). The binding of only one conserved segment is not compatible with our NMR results (c), whereas multivalent binding protects protein partners and the cell. Binding to one partner cannot be ruled out (d), but effective protection is probably achieved by simultaneously binding more than one partner (e).