FIG. 3.
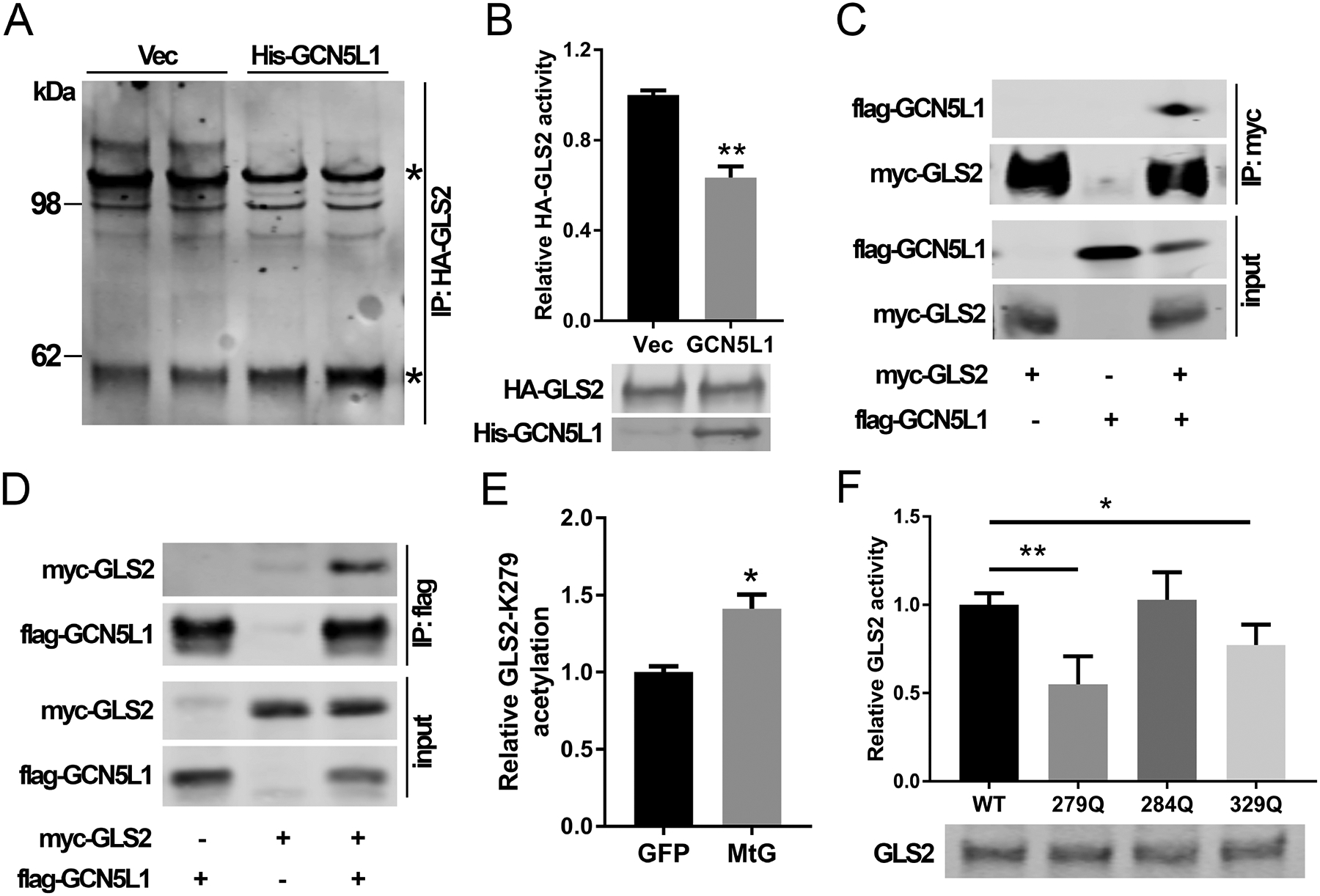
GCN5L1 disrupted GLS2 oligomerization and activity. (A) HA-GLS2 was purified with/without GCN5L1 from 293T cells by pull-down assay using an HA antibody and elution of HA peptides. HA-GLS2 was cross-linked with DSP. Stars indicate GLS2 monomer (~60kDa) and higher molecular weight superstructures. Results are representative of three independent experiments. (B) GLS2 activity was measured using purified HA-GLS2 without crosslinking from 293T cells in the presence acetate and the overexpression of GCN5L1 or a vector control (n = 3 independent experiments). (C and D) GLS2 and GCN5L1 were transfected into 293T cells. Cell lysates were immunoprecipitated with anti-myc (C) or anti-flag (D) antibodies before immunoblotting analysis using anti-flag or anti-myc antibody as indicated. (E) Total acetylated peptides from liver mitochondria of GFP or mitochondrial GCN5L1 expression mice were analyzed by LC-MS. The relative difference in the levels of the only differentiated acetylated GLS2 peptide is shown. (F) WT or mutant GLS2 constructs were transfected into 293T cells. Cell lysates were used to measure GLS2 activities Values are expressed as mean ± s.e.m. *P<0.05, **P < 0.01 versus respective control groups by unpaired Student’s t-test.
