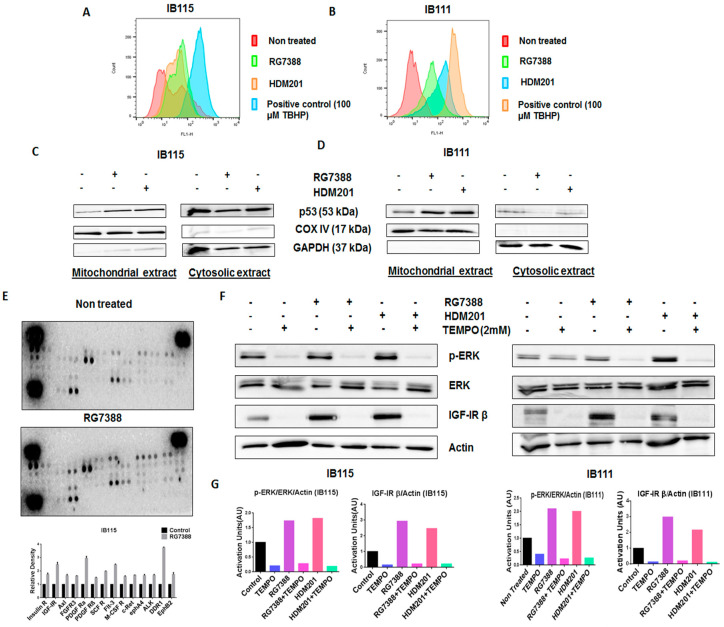
Figure 5.
The mitochondrial translocation of p53 and p53-induced production of reactive oxygen species (ROS) upon treatment with MDM2 antagonists led to the phosphorylation of ERK in DDLPS cells through the activation of receptor tyrosine kinases (RTKs). (A,B) MDM2 antagonists RG7388 and HDM201 induced generation of ROS in IB115 and IB111 cells, which were stained with DCFDA dye for 30 min at 37 °C and treated for 4 h, and the intensities of intracellular DCF were analyzed by flow cytometer. (C,D) MDM2 antagonist (RG7388 and HDM201)-induced generation of ROS is dependent on the mitochondrial translocation of p53. IB115 (C) and IB111 (D) were treated with RG7388 and HDM201 for 24 h. After 24 h, the cells were subjected to subcellular fractionation into cytosolic and mitochondrial fractions for immunoblot analysis of p53. COX IV and GAPDH were used as mitochondrial and cytosolic loading controls, respectively. (E) RG7388 treatment on IB115 cells resulted in an increased expression of Insulin R and PDGFR family RTKs through Phospho-RTK array profiling. Shown is a representative example of phosphoprotein array of control and RG7388-treated IB115 using the Proteome Profiler Human Phospho-RTK Array Kit. This platform allowed simultaneous screening of 49 different RTKs. (F) Effect of TEMPO on RG7388- and HDM201-induced phosphorylation of ERK. DDLPS IB115 and IB111 cells were treated with 2 mM TEMPO either alone or in combination with RG7388 or HDM201 for 24 h. Cells were subjected to immunoblot analysis against p-ERK, ERK, and IGF-1Rβ. Actin was used as the loading control. (G) Quantification of blots of IB115 and IB111 on treatment with TEMPO, RG7388, and HDM201 alone or in combination.