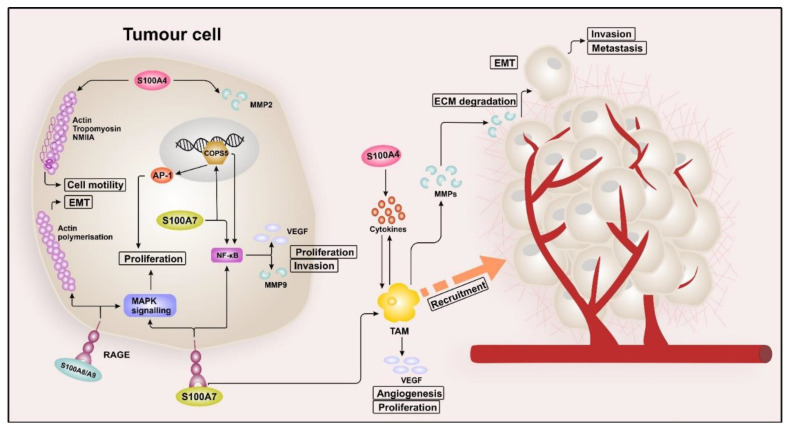
Figure 1.
S100 signalling in breast cancer. Intracellular S100A4 interacts with cytoskeletal proteins, such as actin, non-muscle myosin heavy chain IIA (NMIIA), and tropomyosin, which promotes cell motility. Besides, S100A4 can induce epithelial–mesenchymal transition (EMT) by regulating the expression of matrix metallopeptidases 2 (MMP2), leading to invasion and metastasis. Extracellular S100A4, located in the tumour microenvironment (TME), induces the release of pro-inflammatory factors (e.g., IL-6, IL-8, and CXCL10). These cytokines then convert monocytes into tumour-associated macrophages (TAMs), which in return, promote EMT, proliferation, and drug resistance of the tumour cells. The binding of extracellular S100A7 to the receptor for advanced glycation end products (RAGE) induces mitogen-activated protein kinase (MAPK) and nuclear factor “kappa-light-chain-enhancer” of activated B-cells (NF-κB) signalling, resulting in tumour growth and metastasis. Increased NF-κB activity was observed in S100A7-overexpressing breast cancer cells, associated with evaluated levels of matrix metalloproteinase 9 (MMP9) and vascular endothelial growth factor (VEGF), resulting in proliferation and invasion. The binding of S100A7 and RAGE also leads to the recruitment of TAMs, which then promote further tumour growth, angiogenesis, and metastasis by expressing chemokine (C-C motif) ligand 2 (CCL2), cyclooxygenase-2 (COX2), and VEGF. Intracellular S100A7 interacts with the transcriptional cofactor COP9 constitutive photomorphogenic homolog subunit 5 (COPS5), which in turn promotes the expression of AP-1 and NF-κB, resulting in enhanced tumour growth and invasion. S100A8/S100A9 enhances breast cancer cell growth by inducing MAPK signalling in a RAGE-dependent manner. In addition, RAGE mediates cell migration by promoting actin polymerisation and EMT, leading to metastasis and invasion.