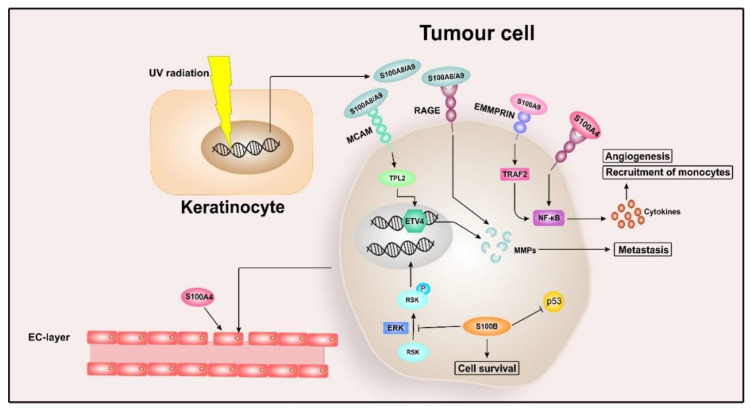
Figure 3.
S100 signalling in melanoma. Extracellular S100A4 binds to the receptor for advanced glycation end products (RAGE) and thereby activates nuclear factor “kappa-light-chain-enhancer” of activated B-cells (NF-κB), resulting in the release of cytokines such as tumour necrosis factor α (TNFα). These cytokines then promote angiogenesis and recruitment of monocytes, creating an inflammatory milieu in the tumour environment. Furthermore, extracellular S100A4 also decreases the expression of occluding and vascular endothelial cadherin (VE)-cadherin in endothelial cells (ECs), thereby disrupting cell–cell adhesion and enabling the tumour cells to transmigrate through the EC monolayer into the bloodstream. The release of S100A8/S100A9 can be induced by UV radiation-exposed keratinocytes, and extracellular S100A8/S100A9 then promotes proliferation and migration of melanocytes via RAGE signalling. The interaction between S100A8/S100A9 and RAGE can lead to increased levels of the metalloproteinases MMP2, MMP9, and MMP14 in melanoma cells, thereby enhancing metastatic properties. In addition to RAGE, S100A8/A9 also binds to the melanoma cell adhesion molecule (MCAM), thereby activating tumour progression locus 2 (TPL2) and stimulating the transcription factor ETS translocation variant 4 (ETV4), leading to the induction of MMP25 and promoting melanoma lung metastasis. The homodimer of S100A9 additionally interacts with extracellular matrix metalloprotease inducer (EMMPRIN), which activates TNF receptor-associated factor (TRAF2)-dependent NF-κB signalling and the upregulation of cytokines such as TNFα, chemokine (C-X-C motif) ligand 1 (CXCL1), CXCL2, and CXCL3, resulting in metastasis. S100B inhibits the phosphorylation of ribosomal S6 kinase (RSK) by extracellular signal-regulated kinase (ERK) in a Ca2+-dependent manner so that RSK remains in the cytoplasm, leading to improved tumour survival. S100B also inhibits p53 activity at the protein level, thereby preventing p53-dependent apoptosis.