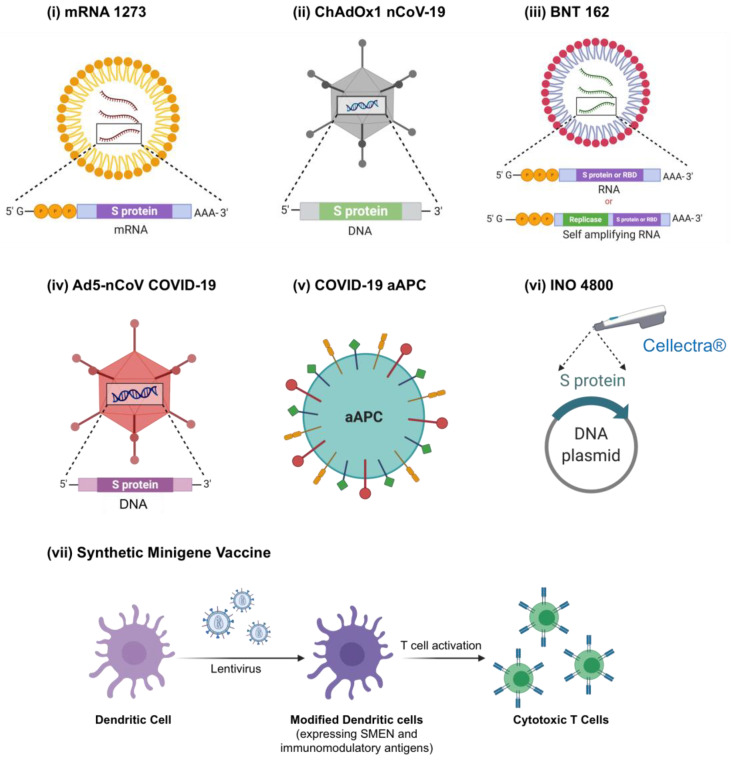
Figure 4.
Vaccine candidates currently in clinical trials for COVID-19. (i) mRNA 1273 is a mRNA-based vaccine, which encodes the full-length S protein of SARS-CoV-2 virus and is delivered via lipid nanoparticles (LNPs). (ii) ChAdOx1 nCoV-19 is a chimpanzee adenovirus vector, which expresses the S protein of SARS-CoV-2 virus inside the host cells and activates the immune system. (iii) BNT 162 is a mRNA-based vaccine delivered via LNPs with four candidates (BNT162a1, BNT162b1, BNT162b2, BNT162c2), encoding either the S protein or receptor binding domain (RBD) of S1 subunit. (iv) Ad5-nCoV COVID-19 is a replication defective adenovirus 5 vector (Ad5) encoding the full-length S protein of SARS-CoV-2 virus. (v) COVID-19 aAPC are artificial antigen-presenting cells (aAPC) modified using a lentivirus vector to express fragments of SARS-CoV-2 proteins and immunomodulatory genes. (vi) INO 4800 is a plasmid DNA encoding SARS-CoV-2 proteins and delivered via electroporation using the smart device Cellectra® developed by Inovio. (vii) Synthetic Minigene Vaccine or LV-SMENP-DC are genetically modified dendritic cells (DCs) via a lentivirus vector to express SARS-CoV-2 minigenes (SMEN) and immunomodulatory genes, administered via subcutaneous injection. Furthermore, T cells activated using the modified dendritic cells are also administered via intravenous infusion.