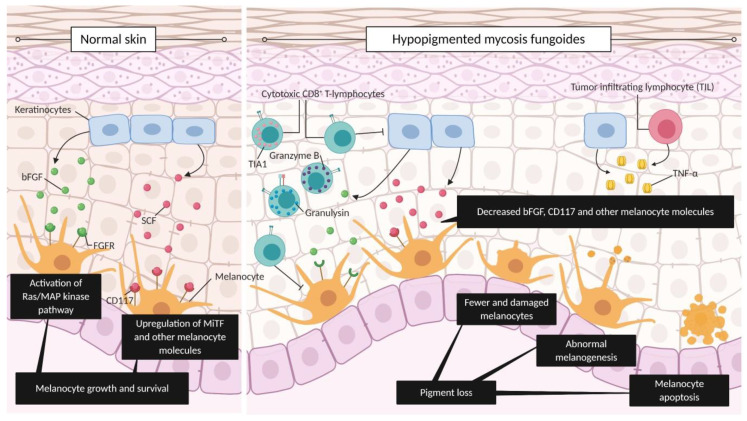
Figure 3.
Hypopigmentation as a surrogate marker of antitumor immune response in mycosis fungoides (MF). In normal skin, keratinocytes produce basic fibroblast growth factor (bFGF) and stem cell factor (SCF). The binding of bFGF to FGF receptor (FGFR) in melanocytes activates the Ras/MAP kinase pathway, while the binding of SCF to its cognate receptor CD177 in melanocytes upregulates microphtalmia-associated transcription factor (MiTF) and additional melanocyte molecules, leading to melanogenesis. Both pathways lead to melanocyte growth and survival. In HMF, cytotoxic CD8+ lymphocytes, which act as part of the antitumor immune response, releasing granulysin and granzyme B, combined with the Th1 inflammatory response rich in TNF-α, result in damage to keratinocytes and melanocytes. This damage to keratinocytes leads to a decreased level of the melanocyte molecules bFGF and CD117, among others. Without the signals needed for melanocyte growth/survival and in the presence of granzyme B, granulysin, and TNF-α, HMF skin eventually has fewer and damaged melanocytes, abnormal melanogenesis, and apoptotic melanocytes. These features lead to the characteristic pigment loss of HMF. Figure created with BioRender.com.