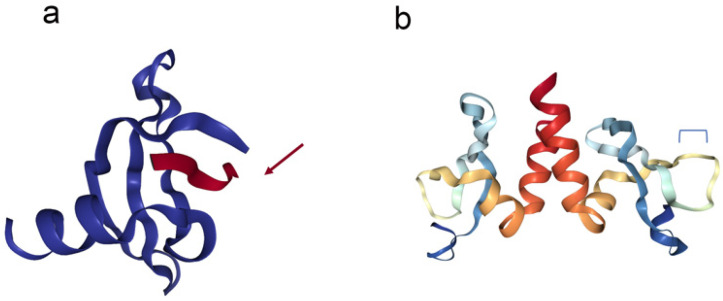
Figure 1.
The crystal structure of the CHD (left) and CSD (right) of HP1a. (a) The left image is a representation of the chromodomain (blue ribbons) of HP1 complexed with histone H3K9me3 from Drosophila (red ribbon mark with red arrow). The CHD (69 aa in length), is made up of three β-sheet antiparallel chains flanked by an α-helix on the C-terminal. The histone tail (16 aa) inserts as a β-strand, completing the β-sandwich architecture of the CHD. (b) On the right side is the CSD with the C-terminal region (rainbow ribbons) of HP1a from Drosophila. The CSD (87 aa) is a dimeric domain and consists of three antiparallel β-sheet chains flanked by two α-helices. The blue bracket represents the interaction site of the PxVxL peptide. Images were created with the PDB (Protein Data Bank) ID 1KNE [36], 3P7J [42], and NGL Viewer [43].