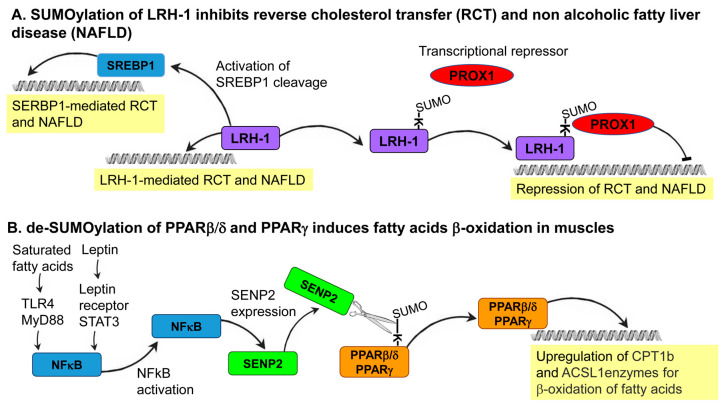
Figure 4.
Two cases-in-point of the regulation of lipid homeostasis by the SUMOylation of nuclear receptors (NRs). (A) In a mouse model system, Liver Receptor Homolog-1 (LRH-1) coordinates programs of reverse cholesterol transport (RCT) and cholesterol metabolism. SUMOyaltion of LRH-1 results in the recruitment of the transcriptional-repressor PROspero-related homeoboX 1 (PROX1) to the LRH-SUMO protein. The LRH/PROX1 complex inhibits the expression of genes that function in RCT and in cholesterol and bile acid excretion [29]. UnSUMOylated LRH-1 also promotes SREBP1 activation and the execution of lipogenesis programs in the liver, which leads to the development of nonalcoholic fatty liver disease [66]. (B) In the mouse skeletal muscle-derived cell line, C2C12, the supplementation of saturated fatty acids activates the toll-like receptor 4 (TLR4) and its adaptor MyD88 [31]. This activation results in NFkB-mediated upregulation of the SUMO protease SENP2, which sheds SUMO from PPARβ/δ and PPARγ. This deconjugation leads to the transcriptional upregulation of fatty-acid oxidation-associated enzymes, such as carnitine palmitoyl transferase-1 (CPT1b) and long-chain acyl-CoA synthetase 1 (ACSL1). SENP2 upregulation was also demonstrated following the treatment of C2C12 cells with the hormone leptin that activates the STAT3 transcription factor [71]. In SENP2 knockout mice, leptin treatment does not upregulate CPT1b and ACSL1 levels as well as fatty acid β-oxidation, demonstrating the in vivo significance of the link between the SUMOylation of PPAR proteins and lipid metabolism [71].