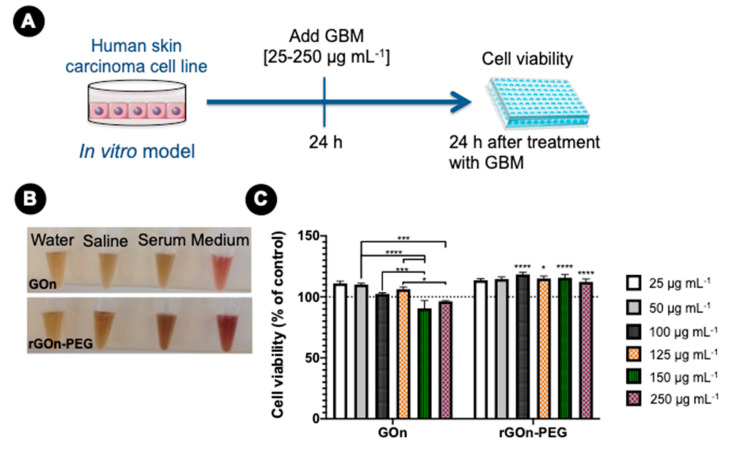
Figure 7.
GBM biocompatibility. (A) Experimental set-up. One day (24 h) after seeding, human skin carcinoma cells (A-431) were treated with different concentrations of GOn and rGOn-PEG and incubated for an additional 24-h period, prior to resazurin assay. (B) GOn and rGOn-PEG (250 μg mL−1) in water and physiological solutions. (C) Cellular viability determined using resazurin assay. Results are normalized with respect to values of the control without GBM. Statistically significant differences are shown as * p < 0.05, *** p < 0.001, **** p < 0.0001. Dashed line represents 100% cell viability of the control without GBM.