Figure 3.
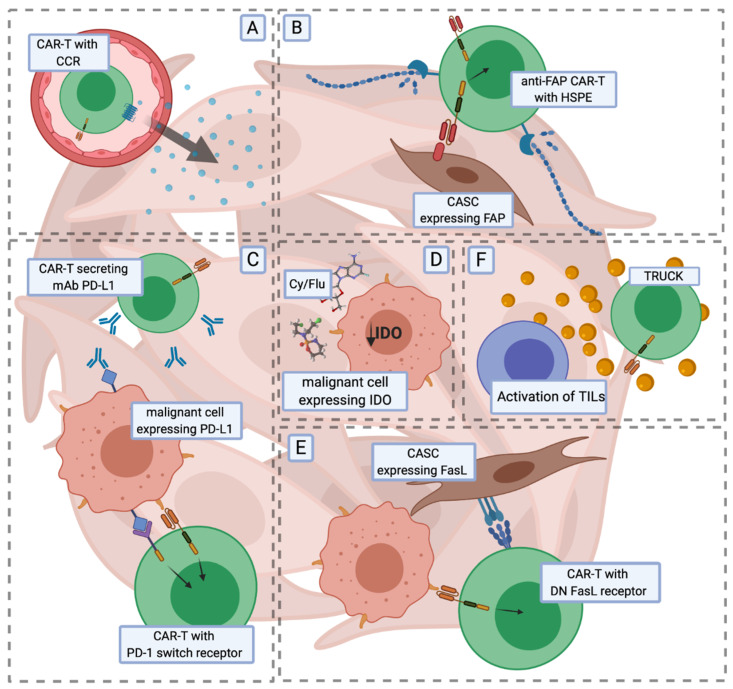
Examples of preclinical concepts supporting CAR-T cell anti-tumour activity in solid tumours. The effective eradication of solid tumours by CAR-T cells is jeopardised by impaired trafficking and infiltration into the tumour mass (A,B), competition with inhibitory signals (C) and reduced survival within the TME. (A) CAR-T cells additionally co-modified with a chemokine receptor (CCR) migrate towards and infiltrate solid masses in response to the chemokine gradient. (B) Forced expression of heparanase (HSPE) and redirection of CAR-T cells against fibroblast activation protein (FAP) further enhances the infiltration of solid masses. Blocking inhibitory molecules, such as PD-L1, by CAR-T cells secreting antibodies (C, top) or modified with switch receptors (C, bottom) providing activation signals leads to increased anticancer efficacy of the therapy. (D) Preconditional therapy with cyclophosphamide and fludarabine (Cy/Flu) decreases the expression level of IDO that inhibits T cell functions. (E) Modification of CAR-T cells with dominant-negative (DN) receptors for FasL leads to the resistance of CAR-T cells to proapoptotic signals present in the TME. (F) Armoured CARs (TRUCKs) engineered to secrete proinflammatory cytokines have enhanced anticancer efficacy and increase the activation of tumour infiltrating lymphocytes (TILs). Abbreviations: TME—tumour microenvironment, PD-L1—programmed death-ligand 1, IDO—indoleamine 2,3 dioxygenase, TRUCK—T cells redirected for universal cytokine killing, CASC—cancer-associated stromal cell, PD-1—program cell death 1, FasL—Fas ligand.