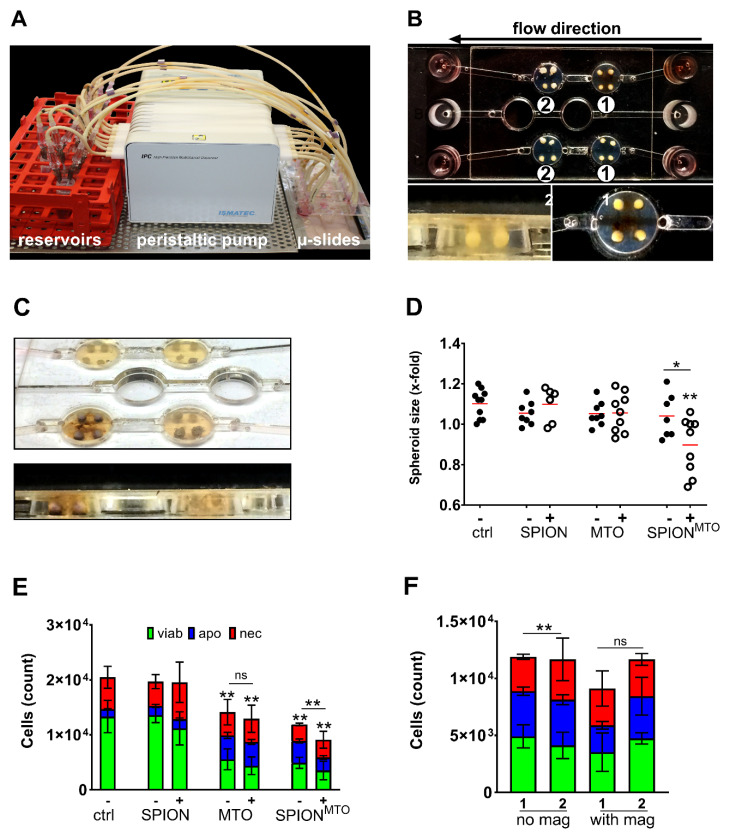
Figure 5.
Magnetic accumulation of SPIONMTO in spheroids under dynamic flow conditions. (A) Experimental setup. A peristaltic pump transported 3 mL of medium through the Ibidi µ-slides at a constant flow rate of 0.5 mL/min. (B) HT-29 spheroids were added in holes pierced into the agarose coating of the flow slides. Magnets were positioned under the first wells of a row in the slides. (C) SPION deposits were visible around spheroids after magnetic accumulation. No change in color was observed in wells treated without magnet. (D) Sizes of the spheroids on day 4 after treatment with SPION, MTO or SPIONMTO +/− magnet. Mock treated cells served as controls. Sizes were normalized to the spheroid sizes before treatment. (E) AnnexinA5-FITC/propidium iodide (Ax/PI) staining of monocell suspensions prepared from spheroids on day 4 after treatment. (F) Comparison of cell counts (Ax/PI staining) between first and second well in serial flow. Two separated circulation systems (no magnet/with magnet) consisted of two wells in serial flow (1/2), each containing 4 spheroids exposed to SPIONMTO. In the circulation system including a magnetic field, the magnet was positioned under only the first well (“1” in group “with magnet”). Well 2 was without magnet. Experiment was performed in two independent experiments each with four spheroids per condition. Shown are the mean values with standard deviations. Significances were calculated for total cell counts using Student’s t-test (* p ≤ 0.05, ** p ≤ 0.01, control versus treated samples or with versus without magnet). Abbreviations: Ax: Annexin A5; FITC: fluorescein isothiocyanate; MTO: mitoxantrone; PI: propidium iodide; SPIONs: superparamagnetic iron oxide nanoparticles; SPIONMTO: mitoxantrone-loaded superparamagnetic iron oxide nanoparticles.