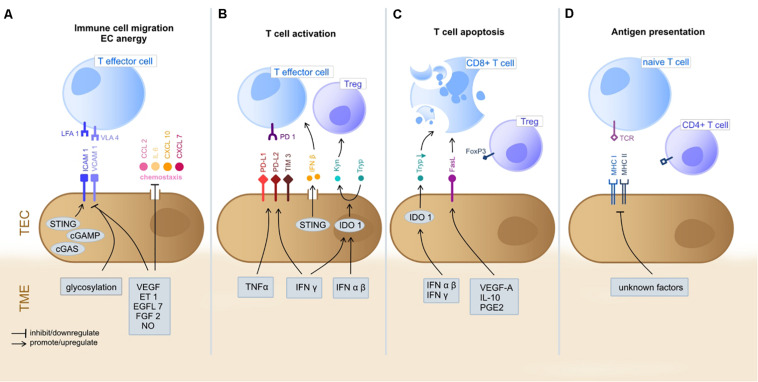
FIGURE 1.
Immunoregulatory functions of TEC in the TME. The gray boxes indicate TME-derived factors that inhibit/promote expression of mediators by TEC. (A) T cell extravasation into the TME starts with a multi-staged adhesion process and includes binding of integrins LFA1 and VLA4 on T cells to the respective ligands ICAM1 and VCAM1 on TEC (Ley et al., 2007; Georganaki et al., 2018). TEC can actively downregulate gene expression of adhesion molecules (e.g., ICAM1 and VCAM1) or chemostaxis themselves in order to control immune cell infiltration (Griffioen et al., 1996; Lambrechts et al., 2018). TME deriving cytokines (e.g., VEGF, ET1, EGFL7, and FGF2) inhibit TEC to upregulate expression of adhesion molecules and chemoattractants (e.g., CCL2, IL6, CXCL10, and CXCL7) (Flati et al., 2006; Buckanovich et al., 2008; Delfortrie et al., 2011). Also, NO has been shown to inhibit adhesion molecule and cytokine (IL6, IL8; not shown) expression (De Caterina et al., 1995). The intracellular cGAS-cGAMP-STING pathway is known to enhance adhesion molecule and promote T cell infiltration (Demaria et al., 2015). The glycosylation of surface molecules (referred to as glycocalyx) modulates the adhesive properties of TEC and can either enhance or reduce immune cell migration (Chandler et al., 2019). (B) T cell activation starts at the TEC/TME interface. Binding of inhibitory immune checkpoints (e.g., PD-1) on CD8+ cells with their ligands (e.g., PD-L1 and PD-L2) on TEC inhibits T cell activation and these ligands can be upregulated by TEC on proinflammatory TME derived factors (IFNγ and TNFα) (Georganaki et al., 2018). STING (see above) acts T cell activating via IFNβ secretion (Demaria et al., 2015). The immunosuppressive cytosolic protein IDO1 promotes the metabolism of Tryp to Kyn. Kyn downstream metabolites promote Treg activation, whilst Tryp depletion promotes T cell apoptosis and inhibits T cell proliferation. TME derived type I IFN (alpha/beta) upregulate IDO1 expression by TEC (Munn and Mellor, 2016; Georganaki et al., 2020). (C) T cell apoptosis can be triggered by IDO1 depended depletion of Tryp (Georganaki et al., 2020). Moreover, the molecule FasL expressed by TEC promotes CD8+ T cell apoptosis whilst sparing Treg (due to expression of FoxP3) and is FasL expression is upregulated in response to TME derived VEGF-A, IL-10 and PGE2 (Motz et al., 2014). TEC, tumor endothelial cells; TME, tumor microenvironment; LFA1, lymphocyte function-associated antigen 1; VLA4, very late antigen-4; ICAM1, Intercellular Adhesion Molecule 1; VCAM1, Vascular cell adhesion protein 1; VEGF, Vascular Endothelial Growth Factor; ET-1, Endothelin-1; EGF-like domain-containing protein 7; FGF2, Fibroblast Growth Factor 2; NO, nitric oxide; PD-1, programd death receptor 1; PD-L1/2, programed death receptor ligand 1/2; Tryp, tryptophan; Kyn, kynurenine; IFN, interferon; IDO1, indoleamine 2,3-dioxygenase 1; MHC, major histocompatibility complex; FasL, Fas ligand. (D) TEC can present processed antigens to T cells via MHC I and MHC II molecules, yet they are lacking the co-stimulatory markers CD80 and CD86 required for naïve T cell activation, distinguishing them from professional APC (Kambayashi and Laufer, 2014). Contrarily, CD4+ T cells are not dependent on co-stimulation at may directly be activated by TEC (Shiao et al., 2007). TEC can actively downregulate HLA genes as immune evading strategy (Goveia et al., 2020), yet external TME-derived factors have not been specified yet.