Abstract
Background: Epilepsy is a common symptom of brain tumors and is often pharmacoresistent. Among new antiseizure medications (ASMs) Brivaracetam (BRV) has been approved as adjunctive treatment for focal seizures and it was tested in non-oncological patient populations. This is the first study that retrospectively explored efficacy and tolerability of BRV as add-on therapy in brain tumor-related epilepsy (BTRE) patients.
Materials and Methods: We reviewed the medical records of 33 BTRE patients from six Italian epilepsy centers; charts included tumor history, diagnosis of BTRE, BRV added as first or second add-on for uncontrolled seizures and/or adverse events (AEs) of the previous ASMs, at least 1-month follow-up, seizure frequency, and AEs assessment.
Results: Thirty-three patients (19 males, mean age: 57.6 years; 14 females, mean age: 42.4 years): 11 low grade gliomas, five high grade gliomas, six meningiomas, 10 glioblastomas, one primary cerebral lymphoma. Fourteen patients had focal aware seizures, nine focal unaware, seven focal to bilateral tonic-clonic seizures, three patients presented more than one seizure type: focal unaware with focal to bilateral tonic clonic seizures (two patients) and focal aware and unaware seizures (one patient). Mean seizure frequency in the month preceding BRV introduction: 7.0; at last follow-up: 2.0 (p = 0.001). Seven patients (21.2%) reported AEs (anxiety, agitation, fatigue, vertigo) and three of them (9.0%) required drug withdrawal due to psychiatric adverse events (PAEs). Three other patients withdrew BRV: one for scarce compliance (3.0%), two for uncontrolled seizures (6.0%).
Conclusion: Our results showed that BRV could be a new therapeutic option effective in reducing seizures in BTRE patients, taking into account the incidence of PAEs in this particular population. Future and larger prospective studies are needed.
Keywords: brain tumor-related epilepsy, antiseizure medication, glioma, responder rate, brivaracetam, adverse events
Introduction
Epilepsy is one of the most common symptoms of brain tumors and it is often pharmacoresistent (1).
The selection of the appropriate antiseizure medication (ASM) in brain tumor related epilepsy patients (BTRE) should be driven by multiple factors, which include not only efficacy in the specific type of seizure to be treated but also tolerability and drug-interaction potential (2).
Among new ASMs, brivaracetam (BRV) is a 2-pyrrolidinone derivative that has been approved as adjunctive therapy and monotherapy for focal (partial-onset) seizures in patients with epilepsy in United States and as adjunctive therapy for focal seizures in patients with epilepsy in the European Union (3).
BRV is an analog of Levetiracetam (LEV) and also selectively binds a novel brain specific binding site synaptic vesicles protein 2A (SV2A). In addition, BVR inhibits voltage dependent Na+ currents and reverses the inhibitory effect of negative allosteric modulators of aminobutyric acid (GABA) and glycine induced current (4, 5).
BRV presents a favorable pharmacokinetic profile, linear and predictable, with low intersubjective variability and almost 100% bioavailability (6). BRV is extensively metabolized through several metabolic pathways and is fully excreted by urine (only 8–11% remains unchanged). The efficacy of BRV as add-on therapy in non-oncological patients with uncontrolled focal seizures has been assessed in six randomized placebo-controlled trials (7–12). The most common adverse events (AEs) reported in literature include: fatigue, dizziness, somnolence, which apparently disappear during treatment (8); moreover a low incidence of neurobehavioral and cognitive AEs is reported (8, 13).
To date, there are no data on efficacy and tolerability of BRV in BTRE patients. However, the choice of antiseizure treatment in this patient population must also take into account the potential interactions between ASMs and chemotherapy and/or support therapies (i.e., corticosteroids) which can induce AEs that may be more frequent than in non-oncological epileptic population (14, 15). Moreover, in this patient population, AEs can affect patient's quality of life more than seizure frequency (16).
Finally, our previous study showed that BRV in vitro exerts a dose-dependent cytotoxic effect on various glioma cell lines, and this effect was concomitant with the modulation of a number of micro RNA (miRNAs) (17), which has been identified in previous studies as predictive marker of seizure occurrence (18) and tumor progression (19).
The aim of this study is to retrospectively evaluate efficacy and tolerability of BRV as add-on therapy, in BTRE patients treated for at least 1 month in six different Italian Epilepsy Centers.
Materials and Methods
Concept Design
Retrospective study (RS 1332/20, 24/04/2020) on medical charts from six Italian Epilepsy Centers of BTRE patients treated for at least 1 month with brivaracetam as add-on in adherence to current clinical practice (GU 31.03.2008, Determinazione AIFA 20.03.2008). Each center was required to send anonymized data regarding BTRE patients seen from September 2018 to February 2019 and followed for at least 1 month. The caring physician had to record and date the patients' clinical record of all actions taken during follow-up (with particular reference to changes in ASM therapy for ineffectiveness and/or adverse effects and concurrent therapies).
The participating centers adhered to the standard follow-up of BTRE patients, and ASM treatment was chosen based on the guidelines of the International League Against Epilepsy (ILAE) (20). All data were collected and merged through an anonymous Excel file developed and agreed upon by the participating centers. Control of quality and completeness of collected data were performed before the statistical analyses. Centers were requested to answer specific queries in the event that further clarification was necessary and, to reduce selection bias, all patients present in the centers' archives were screened and all consecutive patients fulfilling the selection criteria were enrolled.
Medical charts had to include the following information: diagnosis of primary brain tumor according to World Health Organization (WHO) (21); type of surgery: biopsy or surgical resection (partial/total); presence of chemotherapy (CT), radiotherapy (RT) and/or corticosteroids before or during the follow-up period: yes or no; diagnosis of structural epilepsy and seizure classification (focal: aware/unaware) according to new ILAE classification (20); type and dosage of ASMs; BRV added as first or second add-on for uncontrolled seizures and/or for adverse effects of previous ASMs; number of seizures in the month preceding BRV introduction (one or more than one seizure per month) and during follow-up; AEs occurred during BRV therapy collected by patients' spontaneous report (22); date of last follow-up.
An “adverse event” (AE) was defined as any unfavorable and unintended sign, symptom, or disease temporally associated with the medical treatment. Symptoms related to tumor progression were not considered to be AEs. All AEs were recorded in our database, and an AE was attributed to a specific ASM if the attending physician had already written in the medical chart that it had to be attributed directly to the drug or if the AE only occurred or aggravated after starting or increasing the dose of ASM. We defined intolerable AE an AE that led to a decrease in dose or cessation of an ASM. We categorized AEs according NCI-CTCAE as: psychiatric (PAEs) (sedation, agitation, anxiety, irritability), central nervous system (CNSAEs) (vertigo, fatigue), and defined their severity according to NCI-CTCAE classification as grade 1–5 (mild, moderate, severe, life threatening consequences, death) (23).
Primary Aim
To retrospectively evaluate efficacy on seizure control and tolerability of BRV as add-on therapy in BTRE patients.
Secondary Aim
To retrospectively detect the incidence of BRV-related side effects during follow-up period compared with baseline.
Primary Efficacy Variable
Efficacy of BRV was assessed comparing mean seizure frequency at basal visit and at last follow-up available, after each patient reached minimal effective dose of 50 mg/die.
Secondary Efficacy Variables
BRV related side effects at last follow-up available compared to baseline.
Statistical Methods
We computed descriptive statistics for all variables of interest. Continuous data were reported as the mean and standard deviation and we represented categorical data with frequencies and percentage values. In order to investigate the relationships between categorical variables, the Pearson's Chi-squared test and the Fisher Exact test were employed as appropriate. For continuous variables Student's t-test or Mann-Whitney test were used. All statistical analyses were performed with SPSS statistical software version 20 (SPSS Inc., Chicago IL, USA). It was calculated that 24 patients allowed us to evaluate a mean reduction in monthly seizure frequency at about 60% of the standard deviation, assuming a level of significance of 5% and a power of 80%. For each patient we extrapolated the number of seizure in the months immediately before the introduction of brivaracetam from the medical chart, and we compared it with the monthly seizure frequency until the last observation.
Results
We reviewed medical charts of 33 BTRE patients (19 males, mean age: 57.6 years; 14 females, mean age: 42.4 years) from six Italian Epilepsy Centers, followed from 2 to 48 months (mean follow-up duration 10 months), between September 2018 and February 2019. Two patients of our sample had a shorter follow-up, for occurrence of PAEs that led to drug's withdrawal (2 months) and for disease progression (3 months).
Eleven patients (33.3%) had low grade glioma (LGG), 5 (15.2%) high grade glioma (HGG), 6 (18.2%) meningiomas (MEN) (including 1 anaplastic meningioma), 10 (30.3%) glioblastoma (GBM), and one (3.0%) primary cerebral lymphoma (LYM). Tumor site was frontal lobe in 13 patients, temporal lobe in 12, parietal lobe in five, and multilobular in three. Six patients (18.2%) were IDH1 mutated; 11 (33.3%) were negative; 16 (48.5%) were unknown. Twelve patients (36.4%) were MGMT metilated; 1 (3.0%) was unmetilated; in 20 patients (60.6%) the methylation status was not known. Twenty patients underwent gross total resection, 10 partial resections, two biopsies, and one did not undergo surgery; during follow-up nine, patients underwent chemotherapy and one patient underwent radiotherapy (see Table 1). Disease progression during BRV treatment indicated by available neuroradiological data was observed in 11 patients (33.3%).
Table 1.
Patients' clinical and vital data.
| Pat | Age (range) | Sex | Histology | Site of tumor | Surgery | Chemotherapy | RT | Seizure type | No. of seizures in the month before entering the study | Months of follow-up available | ASMs before BRV | ASMs during BRV | BRV dose assigned (mg/day) | No. of seizures/month at last follow-up available | Adverse events during BRV therapy | Disease progression during BRV follow-up | Drop out |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 45-49 | F | MEN | Parietal | GTR | No | Yes° | F-B T-C | 8.0 | 2.0 | LCM 400+VPA 800+PB 150 | LCM 300+VPA 800+PB 100 | 100 | 5,0 | Agitation | No | Yes |
| 2 | 40-44 | F | GBM | Temporal | GTR | BEV+TMZ+ OTHER* | Yes° | FA | 2.0 | 12.0 | CBZ 400+LTG 400+CLZ 14 | LTG 400+CLZ 14 | 100 | 2,0 | No | Yes | No |
| 3 | 50-54 | M | LGG | Multilobular | PR | TMZ° | Yes° | F-U+F-B T-C | 10.0 | 48.0 | LEV 3000+LCM 400 | LCM 400 | 200 | 3,0 | No | No | No |
| 4 | 50-54 | F | GBM | Multilobular | PR | TMZ§ | Yes§ | FA+FU | 30.0 | 6.0 | LEV 1500+LCM 100 | LCM 100 | 100 | 0,0 | No | No | No |
| 5 | 75-79 | F | GBM | Frontal | PR | TMZ° | Yes° | F-U+F-B T-C | 4.0 | 8.0 | LEV 2000 | CLZ 20 | 150 | 4,0 | Fatigue | Yes | No |
| 6 | 45-49 | F | LGG | Temporal | GTR | No | No | FA | 3.0 | 6.0 | ZNS 125 | ZNS 125 | 100 | 0,0 | No | No | No |
| 7 | 30-34 | M | LGG | Temporal | PR | No | Yes° | F-B T-C | 28.0 | 11.0 | LCM 200+LEV 3000 | LCM 200 | 200 | 4,0 | Fatigue | No | No |
| 8 | 35-39 | F | HGG | Temporal | GTR | TMZ* | Yes° | FA | 4.0 | 10.0 | LCM 300 | LCM 200 | 100 | 0,0 | Vertigo | No | No |
| 9 | 60-64 | F | GBM | Temporal | GTR | FTM+TMZ° | Yes° | F-B T-C | 8.0 | 10.0 | LCM 200+PRP 8+LEV 1500 | LCM 200+PRP 8 | 200 | 8,0 | Anxiety | No | Yes |
| 10 | 35-39 | F | LGG | Multilobular | PR | No | Yes° | F-B T-C | 3.0 | 8.0 | LCM 200 | LCM 200 | 100 | 0 | No | No | No |
| 11 | 45-49 | M | HGG | Parietal | GTR | TMZ° | Yes° | FA | 15.0 | 6.0 | PRP 6+LEV 2000 | LCM 100+ PRP 2 | 150 | 0 | Anxiety+Agitation | Yes | No |
| 12 | 55-59 | M | MEN | Temporal | GTR | No | No | F-B T-C | 10.0 | 6.0 | ZNS 300+LEV3000+LCM 400+PRP 8 | ZNS 300+LCM 400+PRP 8 | 100 | 15.0 | Uncontrolled seizures | No | Yes |
| 13 | 35-39 | M | GBM | Frontal | PR | TMZ° | Yes° | F-B T-C | 2.0 | 12.0 | LEV 2000+LCM 100 | LCM 200 | 200 | 1,0 | No | Yes | No |
| 14 | 60-64 | M | A-MEN | Frontal | GTR | No | Yes° | FA | 10.0 | 12.0 | CBZ 600+LEV 1500 | CBZ 200 | 200 | 0,0 | No | No | No |
| 15 | 35-39 | M | LGG | Temporal | PR | No | No | FU | 10.0 | 24.0 | CBZ1200+ZNS 250 | CBZ 1200 | 200 | 3,0 | No | No | No |
| 16 | 40-44 | F | LGG | Parietal | GTR | No | No | FU | 10.0 | 30.0 | LEV 2500+ZNS 200 | ZNS 200 | 200 | 0 | No | No | No |
| 17 | 50-54 | M | HGG | Frontal | PR | TMZ° | Yes° | FA | 0 | 18.0 | LEV 3000+LCM 200 | LCM 200 | 200 | 0 | No | No | No |
| 18 | 70-74 | M | LGG | Temporal | BIO | No | Yes° | FU | 30.0 | 12.0 | VPA 1000 | VPA 500 | 200 | 3 | No | No | No |
| 19 | 20-24 | M | GBM | Frontal | GTR | TMZ+FTM* | Yes° | FA | 2.0 | 18.0 | LEV 1500 | CLZ 10 | 200 | 0 | No | No | No |
| 20 | 50-54 | F | GBM | Parietal | PR | TMZ° | Yes° | FA | 2.0 | 14.0 | LEV 2500+LCM 200 | LCM 200 | 200 | 0 | No | No | No |
| 21 | 40-44 | F | LGG | Frontal | GTR | TMZ+OTHER* | Yes° | FA | 2.0 | 2.0 | LEV 1500+ LCM 150 | LCM 150 | 200 | 8 | Uncontrolled seizures | Yes | Yes |
| 22 | 60-64 | M | LGG | Temporal | No | No | No | FU | 1.0 | 36.0 | CBZ 600 | CBZ 200 | 200 | 0 | No | No | No |
| 23 | 20-24 | M | LGG | Temporal | PR | No | No | FU | 4.0 | 10,0 | CBZ 800 | CBZ 800 | 200 | 5,0 | No | No | Yes |
| 24 | 45-49 | M | GBM | Parietal | GTR | TMZ+FTM* | Yes° | FU | 6.0 | 5.0 | LCM 400 | LCM 400 | 150 | 0,0 | No | Yes | No |
| 25 | 40-44 | F | MEN | Frontal | GTR | No | No | FU | 3.0 | 6.0 | OXC 600 | OXC 600 | 200 | 0,0 | Anxiety | No | Yes |
| 26 | 70-74 | M | LYM | Frontal | GTR | OTHER° | No | FA | 3.0 | 6.0 | LEV 2000+ CLZ 20 | CLZ 20 | 200 | 0,0 | No | No | No |
| 27 | 45-49 | M | HGG | Frontal | GTR | TMZ+FTM* | Yes° | FA | 6.0 | 11.0 | OXC 1200+CLZ 10 | OXC 1200+CLZ 10 | 200 | 1 | No | Yes | No |
| 28 | 75-79 | F | MEN | Frontal | GTR | No | No | F-B T-C | 2.0 | 12.0 | LEV 2000+ CLZ 10 | CLZ 10 | 200 | 0 | No | No | No |
| 20 | 70-74 | M | LGG | Temporal | GTR | TMZ° | No | FA | 3.0 | 8.0 | LEV 2000+ CLZ 10 | CLZ 10 | 200 | 0 | No | No | No |
| 30 | 65-69 | M | MEN | Frontal | GTR | No | No | FU | 3.0 | 5.0 | LEV 2000+ CLZ 20 | CLZ 20 | 200 | 0 | No | Yes | No |
| 31 | 60-64 | M | HGG | Temporal | BIO | TMZ° | No | FA | 3.0 | 6.0 | VPA 1500 | VPA 1500 | 200 | 0 | No | Yes | No |
| 32 | 65-69 | M | GBM | Frontal | GTR | TMZ+FTM* | Yes° | FU | 4.0 | 3.0 | LEV 2000+ CLZ 20 | CLZ 20 | 200 | 0 | No | Yes | No |
| 33 | 75-79 | F | GBM | Frontal | GTR | TMZ+FTM* | Yes° | FA | 2.0 | 6.0 | LEV 2000+ CLZ 10 | CLZ 10 | 200 | 0 | No | Yes | No |
Histology: LGG, low grade glioma; HGG, high grade glioma; MEN, meningioma; A-MEN, anaplastic meningioma; GBM, glioblastoma; LYM, primary cerebral lymphoma.
-Surgery: PR, partial resection; GTR, gross total resection.
-Chemotherapy: °before; *before and during follow-up; §during follow-up.
-Type of chemotherapy: TMZ, temozolomide; FTM, fotemustine; BEV, bevacizumab.
-RT, Radiotherapy: °before; *before and during follow-up; §during follow-up.
-Seizure type: FA, focal aware seizures; FU, focal unaware seizures; F-B T-C, focal to bilateral tonic-clonic.
-ASMs (antiseizure medications): LEV, levetiracetam; VPA, valproic acid; CBZ, carbamazepine; OXC, oxcarbazepine; LTG, lamotrigine; TPM, topiramate; ZNS, zonisamide; LCM, lacosamide; PRP, perampanel; CLZ, clobazam.
Fourteen patients (42.4%) had focal aware seizures, 9 (27.3%) focal unaware, 7 (21.2%) focal to bilateral tonic-clonic seizures, three patients presented more than one seizure type: focal unaware with focal to bilateral tonic clonic seizures (two patients; 6.06%) and focal aware and unaware seizures (one patient; 3.03%). Before starting BRV treatment, 11 patients (33.3%) were on ASM monotherapy (valproic acid-VPA: two patients; carbamazepine-CBZ: two patients; oxcarbazepine-OXC: one patient; zonisamide- ZNS: one patient; levetiracetam-LEV: two patients; lacosamide-LCM: three patients); and 22 (66.6%) on polytherapy (see Table 1).
BRV was introduced for AEs of previous ASMs in five patients (15.2%), for uncontrolled seizure in 19 (57.6%), and for both reasons in 9 (27.3%). BRV starting dosage was 25 mg/die; mean dosage at final follow-up was 175 mg/die.
All patients (n = 20) who assumed LEV in mono or polytherapy were switched to BRV.
During BRV therapy 27 patients (81.8%) assumed one ASM in addiction to BRV (valproic acid-VPA: two patients; carbamazepine-CBZ: four patients; oxcarbazepine-OXC: one patient; zonisamide- ZNS: two patients; clobazam-CLZ: eight patients; lacosamide-LCM: 10 patients) and six patients (18.1%) assumed more than one ASM in addition to BRV (see Table 1).
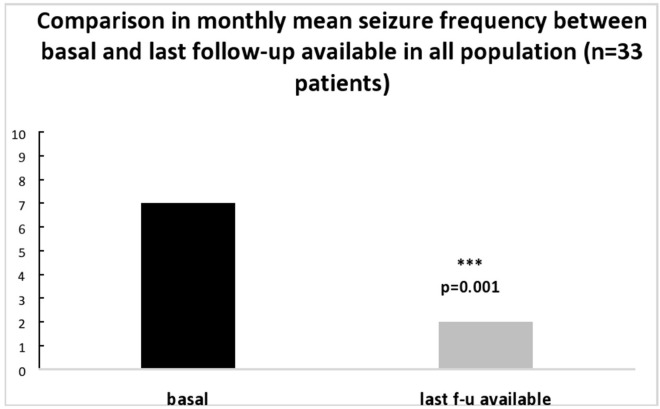
After a mean follow-up of 10 months (duration between 2 and 48 months), monthly mean (±SD) seizure frequency significantly decreased from 7.0 ± 7.9 at basal to 2.0 ± 3.6 at last follow-up available (p = 0.001) (see Figure 1). Twenty patients were seizure free (60.6%), 6 had a reduction ≥50%, one had a reduction ≤50%, 3 were unchanged, 2 patients had an increase in monthly seizure frequency and returned to previous ASM, and one patient shifted to previous ASM due to scarce compliance. The responder rate was 78.7%.
Figure 1.
Comparison in mean seizure number/month between basal and at last follow-up available in all population (n = 33 patients).
We further analyzed differences in BRV efficacy in patients who were switched from LEV to BRV (LEV group; n = 20) vs. patients who did not assume LEV as previous therapy (non-LEV group, n = 13).
We observe similar response at BRV: mean monthly seizure frequency significantly decreased at last follow-up available compared to basal evaluation in both groups (LEV group from 7.5 ± 8.4 to 2.3 ± 4.5; p = 0.01; non-LEV group from 6.4 ± 7.5 to 1.5 ± 1.9; p = 0.03).
Regarding the evaluation of AEs, seven patients (21.2%) out of 33 reported AEs during BRV treatment such as: anxiety (two patients), agitation (one patient), anxiety and agitation (one patient), fatigue (two patients), vertigo (one patient). Among these, patients who experienced PAEs: anxiety (2) and agitation (1) required drug discontinuation (9.0%); one patient (3.0%) with anxiety and agitation had dose reduction with a gradual return to pre-drug conditions; the remaining 3 patients (9.0%) who experienced CNSAEs, such as vertigo or fatigue, ameliorated spontaneously (see Table 2).
Table 2.
Adverse events (AEs) reported in medical charts during BRV treatment.
| Number of patients | % | Action taken | |
|---|---|---|---|
| Anxiety | 2 | 6.0 | Drug's discontinuation |
| Agitation | 1 | 3.0 | Drug's discontinuation |
| Anxiety+Agitation | 1 | 3.0 | Dose reduction |
| Vertigo | 1 | 3.0 | None |
| Fatigue | 2 | 6.0 | None |
We also analyzed whether the incidence of AEs was different in patients who assumed LEV in mono or polytherapy as previous treatment compared to those who did not assumed LEV. We did not observe any significant difference regarding the appearance of BRV related AEs between 20 patients who switched from LEV to BRV (four patients experienced AEs; 20%) and 13 patients without LEV as previous therapy (three patients experienced AEs; 23.0%) (p = 0.78).
In order to assess if BRV efficacy could be influenced by factors related to tumor disease, we compared the number of patients seizure-free vs. patients non seizure-free, in different oncological situations such as: different histology (LGG/HGG; p = 0.28), tumor localization (frontal/temporal/parietal/multilobular; p = 0.16), type of surgery (gross total resection/partial resection; p = 0.11), chemotherapy (yes/no; p = 0.28), radiotherapy (yes/no; p = 0.20), stage of disease (disease progression/stable disease; p = 0.61), IDH1 mutated and non-mutated (p = 0.90); all correlations were not significant. Comparison between patients MGMT-metilated and non-metilated was not evaluable due to the small sample size (12 patients vs. 1 patient).
Discussion
Among the new generation ASMs, BRV is a new therapeutic option in the treatment of drug-resistant epilepsy in adult patients. The drug was tested as adjunctive therapy in different trials only in non-oncological patient populations, from which emerged a good efficacy with a favorable safety profile (7–12, 24–27). This is the first study that explored the efficacy and tolerability of BRV in brain tumor-related epilepsy patients.
We reported the results of retrospective analysis on medical charts of 33 BTRE patients treated with BRV in add-on and followed for a mean of 10 months, between September 2018 and February 2019. BRV treatment was associated with a significant reduction in mean monthly seizure frequency, which decreased from 7.0 ± 7.9 at baseline to 2.0 ± 3.6 at last follow-up available. Responder rate was 78.7% with 20 patients (60.6%) seizure free and 6 patients (18.1%) with a seizure reduction ≥50%. Monthly seizure frequency remained stable in 3 patients and worsened in 2. Literature data from real-life experience studies in non-oncological patients populations with refractory partial epilepsy reported a good efficacy of BRV (25, 26). Steinhoff et al., in a retrospective study on 101 patients treated with BRV in add-on, observed after 6 months of treatment a responder rate of 27.8% with 7% of patients seizure free. Villanueva and colleagues, in a multicenter retrospective analysis on 575 patients, reported a mean reduction in seizure frequency of 36% at 12 months follow-up, with 39.7% of responders and 17.5% of seizure free patients. Even a systematic review and meta-analysis (28) highlighted the effectiveness of BRV as add-on in reducing seizure frequency in adults with drug-refractory non-oncological focal epilepsy. Our results confirm for the first time a good efficacy of BRV in add-on also in BTRE patients, with a high percentage of responders.
Moreover, literature data on adults with drug-refractory non-oncological focal epilepsy (28) reported a greater BRV related-treatment effect in LEV-naïve patients, rather than in patients who previously assumed LEV, in which BRV showed lower efficacy. In our population with BTRE, we observed similar response to BRV both in the sub-group of patients switched from LEV and in the sub-group of patients who did not assume LEV as previous therapy. Regarding BRV tolerability, our results showed the incidence of AEs in 7 patients (21.2%), which consisted of: agitation, anxiety, fatigue, and vertigo. PAEs observed in 4 patients (57.1%) were the main reason for drug discontinuation (2 patients for anxiety, 1 patient for agitation) or reduction (1 patient for anxiety and agitation), while CNSAEs such as vertigo or fatigue (observed in 3 patients) ameliorated spontaneously during treatment. Previous studies (25, 26) on tolerability of BRV in non-oncological patient population reported the presence of AEs such as: dizziness, somnolence, irritability, anxiety, aggression, and depression. Villanueva and colleagues observed an incidence of physical and psychiatric AEs, respectively in 39.8 and 14.3% of cases; the highest percentage of drug discontinuation was due to physical side effects (8.9%). In a retrospective study by Steinhoff et al., incidence of AEs was about 37%, most of which were dizziness and somnolence, with a lower rate of psychiatric ones; the main reason for drug discontinuation was lack of efficacy.
Our results showed in our patients with BTRE a total number of AEs lower than in non-oncological patients. Nevertheless, the incidence of PAEs was higher while the incidence of CNSAEs was not. For this reason, we recommend clinicians to inform the caregivers and patients of possible AEs upon initiating ASMs for BTRE, carefully monitoring their incidence and considering change of therapy if AEs reduce patients' quality of life. Furthermore, in our sample, the appearance of BRV related AEs was not affected by assuming LEV as previous therapy. We did not observe significant differences in AEs occurrence between patients who were switched from LEV to BRV and patients in whom BRV was added, as previously reported by Steinhoff et al. (25), in non-oncological patients population.
Finally, literature data on BTRE patients (29) indicated that a number of factors related to oncological disease (i.e., histology, tumor location, type of surgery, molecular markers) have been associated with a higher seizure risk, but, in our patient, BRV as add-on therapy maintained a good efficacy over time independently by these risk factors.
This study has several limitations. First, this is a retrospective study. Data have been obtained from medical records where, in the absence of standardized and systematic collection of the required information, variables were available in non-standardized format and occasional variables, not directly correlated with the aim of the study, were excluded.
Second, treatment retention was assessed in an observational context. Physicians' and patients' judgment might have had strong influence on the decision to start/stop the assigned treatment. Third, this is a multicenter study. Management of the disease varies across centers and this may have a strong impact on study results. Finally, the small sample size and the relatively short follow-up could have influenced our results. Regarding to this aspect, it has to be considered that patients with epilepsy and brain tumors represent a very fragile population, with a complex clinical profile, poor life expectancy, and a rare disease. For this reason, it was difficult to balance the need to have a sufficiently large sample of patients, with the need to have a statistically homogenous sample size with long follow-up.
All these limitations imply a cautious interpretation of our findings and should encourage future multicenter studies with randomized trial and longer follow-up aimed to further evaluate the role of BRV as add-on therapy in BTRE patient populations.
Conclusion
Despite the limitations of our retrospective study, our results showed that BRV could be a new therapeutic option effective in reducing epileptic seizures in BTRE patients, taking into account the incidence of psychiatric adverse events in this particular patient population.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethical Committee RS 1332/20, 24/04/2020. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
MM: conceptualization and methodology, project administration and supervision, data collection, and writing-review and editing. AMai: data collection and writing-original draft. CM, ED, GP, CC, AMas, and MR: data collection. DG: statistical analysis. All authors contributed to the article and approved the submitted version.
Conflict of Interest
MM has received support for travel to congresses from EISAI srl; has participated in scientific advisory boards for EISAI; has participated in pharmaceutical industry-sponsored symposia for UCB Pharma; has received research grants from UCB Pharma. GP has participated in advisory boards and industry-sponsored symposia for Eisai, UCB, LivaNova. She received speakers' fees from Eisai, LivaNova and UCB in the past 3 years. CC has received research grants from UCB and EISAI; has participated in scientific advisory boards for EISAI; has participated in pharmaceutical industry-sponsored symposia for EISAI. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Thanks to Dr. Selvaggia Camilla Serini for reviewing the manuscript.
References
- 1.Maschio M. Brain tumor-related epilepsy. Curr Neuropharmacol. (2012) 10:124–33. 10.2174/157015912800604470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maschio M, Dinapoli L, Zarabla A, Jandolo B. Issues related to the pharmacological management of patients with brain tumours and epilepsy. Funct. Neurol. (2006) 21:15–19. [PubMed] [Google Scholar]
- 3.Brandt C, Klein P, Badalamenti V, Gasalla T, Whitesides J. Safety and tolerability of adjunctive brivaracetam in epilepsy: In-depth pooled analysis. Epilepsy Behav. (2020) 103(Pt A):106864. 10.1016/j.yebeh.2019.106864 [DOI] [PubMed] [Google Scholar]
- 4.von Rosenstiel P. Brivaracetam (UCB 34714). Neurotherapeutics. (2007) 4:84–7. 10.1016/j.nurt.2006.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zona C, Pieri M, Carunchio I, Curcio L, Klitgaard H, Margineanu DG. Brivaracetam (ucb 34714) inhibits Na(+) current in rat cortical neurons in culture. Epilepsy Res. (2010) 88:46–54. 10.1016/j.eplepsyres.2009.09.024 [DOI] [PubMed] [Google Scholar]
- 6.Rolan P, Sargentini-Maier M, Pigeolet E, Stockis A. The pharmacokinetics, CNS pharmacodynamics and adverse event profile of brivaracetam after multiple increasing oral doses in healthy men. Br J ClinPharmacol. (2008) 66:71–5. 10.1111/j.1365-2125.2008.03158.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.French J, Costantini C, Brodsky A, Von Rosenstiel P, Group N. Adjunctive brivaracetam for refractory partial-onset seizures: a randomized, controlled trial. Neurology. (2010) 75:519–25. 10.1212/WNL.0b013e3181ec7f7f [DOI] [PubMed] [Google Scholar]
- 8.Klein P, Schiemann J, Sperling M, Whitesides J, Liang W, Stalvey T, et al. A randomized, double-blind, placebo-controlled, multicenter, parallel-group study to evaluate the efficacy and safety of adjunctive brivaracetam in adult patients with uncontrolled partial-onset seizures. Epilepsia. (2015) 56:1890–8. 10.1111/epi.13212 [DOI] [PubMed] [Google Scholar]
- 9.Van Paesschen W, Hirsch E, Johnson M, Falter U, von Rosenstiel P. Efficacy and tolerability of adjunctive brivaracetam in adults with uncontrolled partial-onset seizures: a phase IIb, randomized, controlled trial. Epilepsia. (2013) 54:89–97. 10.1111/j.1528-1167.2012.03598.x [DOI] [PubMed] [Google Scholar]
- 10.Ryvlin P, Werhahn K, Blaszczyk B, Johnson M, Lu S. Adjunctive brivaracetam in adults with uncontrolled focal epilepsy: results from a double-blind, randomized, placebo-controlled trial. Epilepsia. (2014) 55:47–56. 10.1111/epi.12432 [DOI] [PubMed] [Google Scholar]
- 11.Biton V, Berkovic S, Abou-Khalil B, Sperling M, Johnson M, Lu S. Brivaracetam as adjunctive treatment for uncontrolled partial epilepsy in adults: a phase III randomized, double-blind, placebo-controlled trial. Epilepsia. (2014) 55:57–66. 10.1111/epi.12433 [DOI] [PubMed] [Google Scholar]
- 12.Kwan P, Trinka E, Van Paesschen W, Rektor I, Johnson M, Lu S. Adjunctive brivaracetam for uncontrolled focal and generalized epilepsies: results of a phase III, double-blind, randomized, placebo-controlled, flexible-dose trial. Epilepsia. (2014) 55:38–46. 10.1111/epi.12391 [DOI] [PubMed] [Google Scholar]
- 13.Meador K, Gevins A, Leese P, Otoul C, Loring D. Neurocognitive effects of brivaracetam, levetiracetam, and lorazepam. Epilepsia. (2011) 52:264–72. 10.1111/j.1528-1167.2010.02746.x [DOI] [PubMed] [Google Scholar]
- 14.Hildebrand J. Management of epileptic seizures. CurrOpin Oncol. (2004) 16:314–7. 10.1097/01.cco.0000127720.17558.38 [DOI] [PubMed] [Google Scholar]
- 15.Hildebrand J, Lecaille C, Perennes J, Delattre JY. Epileptic seizures during follow-up of patients treated for primary brain tumors. Neurology. (2005) 65:212–5. 10.1212/01.wnl.0000168903.09277.8f [DOI] [PubMed] [Google Scholar]
- 16.Maschio M, Dinapoli L, Vidiri A, Pace A, Fabi A, Pompili A, et al. The role side effects play in the choice of antiepileptic therapy in brain tumor related epilepsy: a comparative study on traditional antiepileptic drugs versus oxcarbazepine. J ExpClin Cancer Res. (2009) 28:60. 10.1186/1756-9966-28-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rizzo A, Donzelli S, Girgenti V, Sacconi A, Vasco C, Salmaggi A, et al. In vitro antineoplastic effects of Brivaracetam and Lacosamide on human glioma cell. J Exp Clin Cancer Res. (2017) 36:76. 10.1186/s13046-017-0546-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henshall DC. MicroRNA and epilepsy: profiling, functions and potential clinical applications. CurrOpin Neurol. (2014) 27:199–205. 10.1097/WCO.0000000000000079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet. (2007) 39:673–7. 10.1038/ng2003 [DOI] [PubMed] [Google Scholar]
- 20.Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, et al. Operational classification of seizure types by the international league against epilepsy: position paper of the ilae commission for classification and terminology. Epilepsia. (2017) 58:522–30. 10.1111/epi.13670 [DOI] [PubMed] [Google Scholar]
- 21.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of tumors of the Central Nervous System: a summary. Acta Neuropathol. (2016) 131:803–20. 10.1007/s00401-016-1545-1 [DOI] [PubMed] [Google Scholar]
- 22.Gilliam FG, Fessler AJ, Baker G, Vahle V, Carter J, Attarian H. Systematic screening allows reduction of adverse antiepileptic drug effects. A randomized trial. Neurology. (2004) 62:23–7. 10.1212/WNL.62.1.23 [DOI] [PubMed] [Google Scholar]
- 23.CTEP Active CTCAE Version (Active as of 10/1/2009) Common Terminology Criteria for Adverse Events (CTCAE) v4.0. Available online at: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-0614_QuickReference_5x7.pdf
- 24.Brandt C, May TW, Bien C. Brivaracetam as adjunctive therapy for treatment of partial onset seizures in patients with epilepsy: the current evidence base. Ther Adv NeurolDisord. (2016) 9:474–82. 10.1177/1756285616665564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinhoff BJ, Bacher M, Bucurenciu I, Hillenbrand B, Intravooth T, Kornmeier R, et al. Real-life experience with brivaracetam in 101 patients with difficult-to-treat epilepsy-A monocenter survey. Seizure. (2017) 48:11–4. 10.1016/j.seizure.2017.03.010 [DOI] [PubMed] [Google Scholar]
- 26.Villanueva V, López-González FJ, Mauri JA, Rodriguez-Uranga J, Olivé-Gadea M, Montoya J, et al. BRIVA-LIFE study group. BRIVA-LIFE-A multicenter retrospective study of the long-term use of brivaracetam in clinical practice. Acta Neurol Scand. (2019) 139:360–8. 10.1111/ane.13059 [DOI] [PubMed] [Google Scholar]
- 27.Brodie MJ, Whitesides J, Schiemann J, D'Souza J, Johnson ME. Tolerability, safety, and efficacy of adjunctive brivaracetam for focal seizures in older patients: a pooled analysis from three phase III studies. Epilepsy Research. (2016) 127:114–8. 10.1016/j.eplepsyres.2016.08.018 [DOI] [PubMed] [Google Scholar]
- 28.Lattanzi S, Cagnetti C, Foschi N, Provinciali L, Silvestrini M. Brivaracetam add-on for refractory focal epilepsy. A systematic review and meta-analysis. Neurology. (2016) 86:1344–52. 10.1212/WNL.0000000000002545 [DOI] [PubMed] [Google Scholar]
- 29.ErtürkÇetin Ö, Işler C, Uzan M, Özkara Ç. Epilepsy-related brain tumors. Seizure. (2017) 44:93–97. 10.1016/j.seizure.2016.12.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.