Figure 1: Signaling pathways initiated by the TNF-Receptor superfamily.
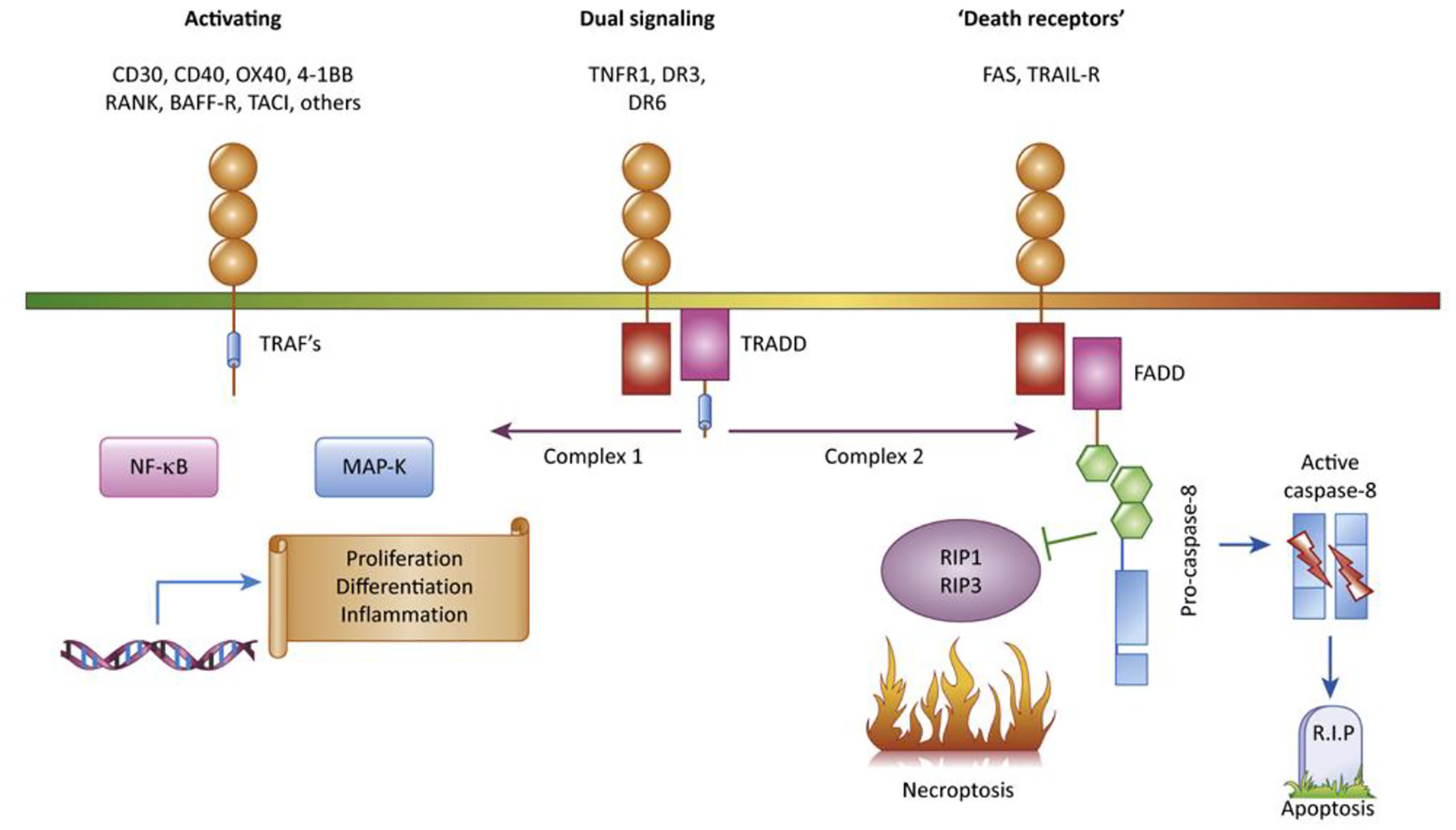
The 29 TNF receptors in the human genome can be divided into three subtypes based on the adapter proteins recruited to their cytoplasmic tails, in addition to ‘decoy receptors;’ that are soluble or lack functional intracellular signaling domains. The largest group of receptors contain peptide motifs in their cytoplasmic domains which recruit TRAF adapter proteins, which activate intracellular signaling pathways culminating in transcriptional responses and production of inflammatory cytokines, proliferation and cellular differentiation. Fas and TRAIL receptors recruit FADD, an adapter protein that mediates recruitment of caspase-8 and can activate apoptosis, with inhibition of necrosis and inflammatory and differentiation pathways as more newly recognized functions. TNFR1, DR3 and DR6 recruit the TRADD adapter protein, which can alternatively activate inflammatory or caspase-mediated signaling.
