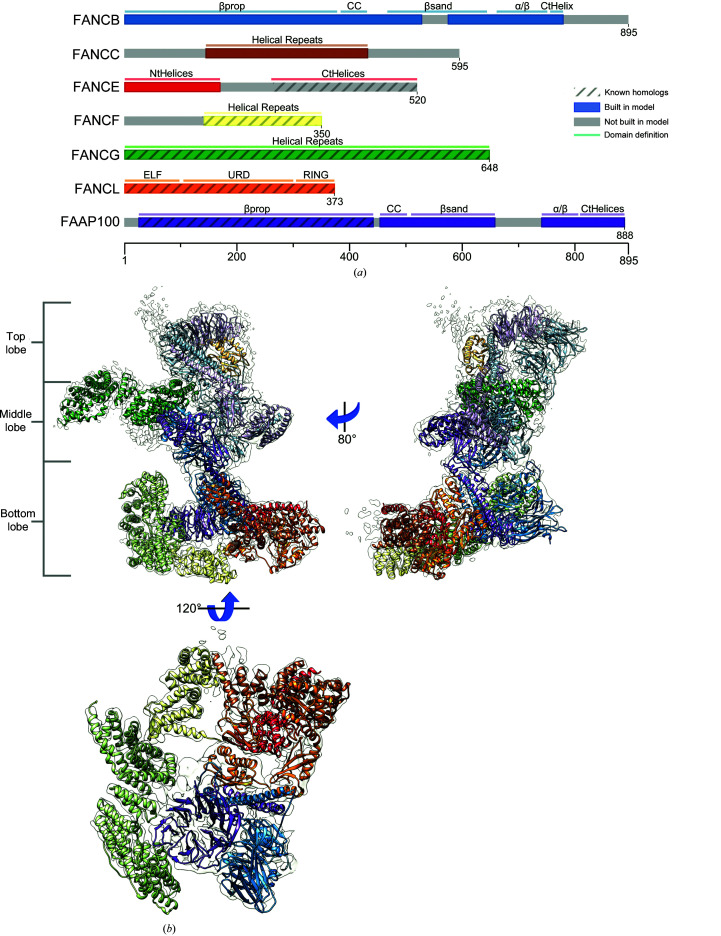
Figure 2.
An overview of FAcc. (a) Domain organization of the seven subunits of FAcc. Based on our modeling, we find that the complex consists of 18 domains, indicated with narrow bars. FANCB and FAAP100 have the same domain organization, with a β-propeller (βprop) followed by a long coiled coil (CC), a β-sandwich (βsand) and then an α/β domain, finally followed by a C-terminal helical region. FANCC, FANCF and FANCG are all comprised of a single helical-repeat domain, while we find FANCE to have two separated helical-repeat domains (one N-terminal and one C-terminal). Finally, FANCL is organized as an ELF domain followed by a URD domain and then lastly a RING domain. Also indicated is the availability of known structures or homologous proteins throughout the modeling process with striations. Domains with known structures or available homologous proteins used include the C-terminal helices of FANCE, the helical repeats of FANCF, all of FANCG and FANCL, and the β-propeller of FAAP100. (b) Three views of the complete model of FAcc as determined by our modeling protocol. Colors are matched to the diagram in (a), with those that have multiple copies (FANCB, FANCG and FAAP100) having different shades of the coloring. The orientations of the top, middle and bottom lobes are indicated.