Abstract
Cyclophosphamide (CP) is one of the most potent alkylating agents and is widely used in the treatment of numerous neoplastic conditions, autoimmune diseases and following organ transplantation. Due to its ability to induce oxidative stress and subsequent apoptosis, CP is affiliated with many adverse effects with special emphasis on the highly prevalent hepatotoxicity. Dipeptidyl peptidase 4 (DDP-IV) inhibitors are being rediscovered for new biological effects due to their ability to target multiple pathways, among which is the phosphoinositide 3–kinase (PI3K) and protein kinase B (Akt) axis. This could offer protection to multiple organs against reactive oxygen species (ROS) through modulating sirtuin 1 (SIRT1) expression and, in turn, inactivation of forkhead box transcription factor of the O class 1 (FoxO1), thus inhibiting apoptosis. Accordingly, the current study aimed to investigate the potential therapeutic benefit of alogliptin (Alo), a DPP-IV inhibitor, against CP-induced hepatotoxicity through enhancing PI3K/Akt/SIRT1 pathway. Forty male Wistar rats were randomly divided into four groups. The CP-treated group received a single dose of CP (200 mg/kg; i.p.). The Alo-treated group received Alo (20 mg/kg; p.o.) for 7 days with single CP injection on Day 2. Alo successfully reduced hepatic injury as witnessed through decreased liver function enzymes, increased phospho (p)-PI3K, p-Akt, superoxide dismutase (SOD) levels, SIRT1 expression, p-FoxO1 and anti-apoptotic B-cell lymphoma 2 (Bcl-2). This resulted in decreased apoptosis, as witnessed through decreased caspase-3 levels and improved histopathological picture. In conclusion, the current study succeeded to elaborate, for the first time, the promising impact of Alo in ameliorating chemotherapy-induced liver injury.
Keywords: cyclophosphamide, hepatotoxicity, alogliptin, SIRT1, FoxO1
Graphical Abstract
Graphical Abstract.
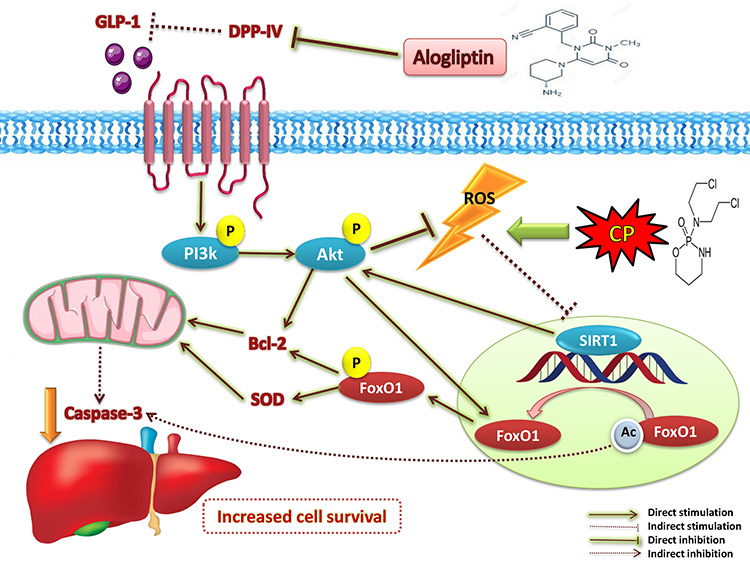
Introduction
Cyclophosphamide (CP) is one of the most potent alkylating agents and is widely used in the treatment of numerous neoplastic conditions [1, 2], autoimmune diseases [3] and following organ transplantation [4]. Due to its ability to induce oxidative stress and subsequent cellular apoptosis, CP is affiliated with genotoxicity and many adverse effects on multiple organs, with special emphasis on the highly prevalent hepatotoxicity [5–7]. Synchronously, sirtuin (SIRT) dysfunction arises as a prominent pathophysiological factor in the molecular pathogenesis of oxidative stress-induced cell death [8]. Vice versa, SIRT1 hinders Bax-mediated apoptosis [9] and enhances the cellular anti-oxidant activity [10], thus alleviates oxidative stress and inflammation, and improves mitochondrial biogenesis, which all contribute to enhanced cellular longevity. SIRT1 favorable effects are attributed to its ability to deacetylate multiple substrates [11]. Among these substrates is forkhead box transcription factor of the O class (FoxO), where its deacetylation by SIRT1 allows its translocation outside the nucleus and phosphorylation, and in turn, inactivated [12]. Thus, enhancing SIRT expression can be regarded as a promising candidate to combat chemotherapy-associated organ toxicity.
On the other hand, the phosphoinositide 3–kinase (PI3K) and protein kinase B (Akt) signaling axis exerts a pivotal role in regulating cell proliferation and survival in response to various stimuli [13]. Independently from SIRT1, this axis mitigates FoxO gene transcription [14, 15]. Furthermore, PI3K/Akt pathway can indirectly enhance SIRT1 action through reducing oxidative stress [16]. These findings raise temptations to find therapeutic molecules that can target PI3K/Akt and SIRT1 simultaneously to increase cell survival.
Alogliptin (Alo), a dipeptidyl peptidase 4 (DDP-IV) inhibitor, is mainly used as an antidiabetic agent [17]. Interestingly, gliptins are being rediscovered for new biological effects owing to their ability to target multiple pathways via increasing glucagon-like peptide 1 (GLP-1) levels [18]. Increased GLP-1 levels can activate the downstream PI3K/Akt signaling pathway, and subsequently, phosphorylate several substrates, such as FoxO1 [19]. Moreover, gliptins revealed protective effects through triggering 5′ AMP-activated protein kinase/SIRT1 signaling pathway. This resulted in reduced production of reactive oxygen species (ROS) and abridged mitochondrial dysfunction [20]. Therefore, the current study aimed to investigate the potential therapeutic benefit of Alo against CP-induced hepatotoxicity via tracking the PI3K/Akt/SIRT1/FoxO1 pathway and their impact upon liver function enzymes, cellular apoptosis and oxidative stress, as well as the liver histological architecture.
Materials and Methods
Ethics statement
The study was conducted in agreement with the ethical procedures and policies approved by the Institutional Review Board of Faculty of Pharmacy, Misr International University, Cairo, Egypt, and conforms with the ARRIVE guidelines [21] and Guide for the Care and Use of Laboratory Animals [22]. All efforts were made to minimize animal suffering and to decrease the number of animals used.
Animals
Forty adult male Wistar rats (200 ± 20 g) were purchased from The Nile Company for Pharmaceuticals and Chemical Industries (Cairo, Egypt). Rats were allowed 1-week acclimatization period at the animal facility of Faculty of Pharmacy, Misr International University, in standard polypropylene cages (three rats per cage). They were allowed free access to normal pellet diet (EL Nasr Pharmaceutical Chemicals Co., Cairo, Egypt) and tap water throughout the experimental period. The rats were kept under standard conditions of temperature (22 ± 2°C) and relative humidity (55 ± 5%) with 12-light/12-dark cycle.
Experimental design
Alogliptin (Sigma-Aldrich, Missouri, USA) was suspended in 0.5% carboxymethyl cellulose (CMC) sodium (0.5 g in 100 ml H2O) (MP Biomedicals, California, USA) to reach a final concentration of 2 mg/ml [23]. Cyclophosphamide (Endoxan®, Baxter Oncology GmbH, Halle Westfalen, Germany) was dissolved in normal saline 0.9% to a final concentration of 2% (20 mg/ml).
Rats were randomly divided into 4 groups, each containing 10 rats as follows:
Group 1: rats received 0.5% CMC (10 ml/kg; p.o.) for 7 days, with single dose of injection of saline (10 ml/kg; i.p.) on Day 2 to serve as control.
Group 2: rats received Alo (20 mg/kg; p.o.) for 7 days, with single dose injection of saline (10 ml/kg; i.p.) on Day 2 [23].
Group 3: rats received 0.5% CMC (10 ml/kg; p.o.) for 7 days, with CP single dose injection on Day 2 (200 mg/kg; i.p.) [24].
Group 4: rats received Alo (20 mg/kg; p.o.) for 7 days, with CP single dose injection on Day 2 (200 mg/kg; i.p.).
Serum and tissue collection: on Day 8, after overnight fasting, all animals were anesthetized with a cocktail of i.p. ketamine hydrochloride (50 mg/kg) and xylazine (5 mg/kg) [25] purchased from Sigma-Aldrich (Missouri, USA), then blood samples were collected from retro-orbital venous plexus by capillary tubes. The blood was then centrifuged at 3000 rpm for 15 minute for serum collection. Serum was separated and stored frozen at −80°C until analysis for assay of liver function enzymes. Lastly, all rats were sacrificed then livers were quickly dissected and divided in each group into two subsets; the first subset comprised eight livers that were divided into portions and immediately stored at −80°C until assessment of biochemical markers. The second subset comprised two livers that were rapidly flushed, fixed in 10% neutral buffered formalin for 72 hours and processed for light microscopical examination. All assessments were carried out by blinded investigators.
Methods
Assessment of liver function enzymes
Serum alanine transaminase (ALT) and aspartate transaminase (AST) were determined using the corresponding Stanbio colorimetric assay kits (Liqui-UV; Texas, USA).
Assessment of hepatic superoxide dismutase, Bcl-2 and caspase-3
Rat sandwich ELISA kits were purchased from MyBioSource (California, USA) to assess the apoptotic markers, Bcl-2 (catalog # MBS704498) and caspase-3 (catalog # MBS261814). Similarly, rat ELISA kit for assay of superoxide dismutase (SOD) (catalog # CSB-EL022397RA) was purchased from Cusabio (Wuhan, P.R. China). All procedures were done according to the manufacturers’ instructions.
Assessment of hepatic gene expression of SIRT1
Total RNA was extracted from liver tissue homogenate using RNeasy Kit (Qiagen, Hilden, Germany), and the tissue lysate was centrifuged for 3 minutes at 10,000 × g, then the supernatant was taken. The supernatant was reverse transcribed into cDNA using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, California, USA) according to the manufacturer’s guidelines. To assess the expression of SIRT1 gene, qPCR was performed using SYBR® Green PCR Master Mix (Applied Biosystems, California, USA) as described by the manufacturer. The relative expression of SIRT1 gene was obtained using the ΔΔ CT method as previously described [26] using GAPDH as a housekeeping gene (Table 1).
Table 1.
Primer sequences used for RT-qPCR
| Gene | GenBank accession number | Forward primer | Reverse primer |
|---|---|---|---|
| SIRT1 | XM_017601788 | GGCAGACAATTTAATGGGGTGA | GAGATCCGGGAAGTCCACAG |
| GADPH | NM_017008 | GTTACCAGGGCTGCCTTCTC | GATGGTGATGGGTTTCCCGT |
Assessment of protein levels of phosphorylated PI3K (Tyr607), Akt (Thr450) and FoxO1 (Ser249)
Samples of equal protein concentrations (≈20 μg) were electrophoresed using 10% sodium dodecyl sulfate/polyacrylamide gel (SDS/PAGE) and electro-transferred to polyvinylidene difluoride membranes. The membranes were blocked with 5% (w/v) skimmed milk powder in PBS/Tween-20 for 2 hours at room temperature. Then, the membranes were incubated with p-PI3K (Tyr607) [Thermo Fisher Scientific, catalog # PA5-38905, RRID AB_2555497], p-Akt (Thr450) [Thermo Fisher Scientific, catalog # PA5-37469, RRID AB_2554078] and p-FoxO1 (Ser249) [Thermo Fisher Scientific, catalog # PA5-64676, RRID AB_2661988] polyclonal antibodies (1:1000) diluted in tris-buffered saline-tween containing 1% bovine serum albumin and β-actin (Santa Cruz Biotechnology) as internal control diluted 1:1000 in blocking buffer. The membranes were incubated with the corresponding secondary antibodies for 1 hour at room temperature, washed and then developed. Finally, images of indicated protein bands were recorded on the BioMax film (Kodak), and densitometrical quantification was conducted by using Image J software (Bio-Rad, California, USA). Densities of bands were standardized to the corresponding density of β-actin.
Protein content
Tissue protein concentration in liver tissue samples was estimated as described by Bradford [27].
Light microscopic examination
After fixation of the liver, the tissues were washed in several changes of 70% ethanol, followed by dehydration in ascending grades of alcohol, clearing in xylene and embedding in paraffin wax to obtain paraffin blocks [28]. Sections of 5 μm thickness were cut, mounted on slides and stained with hematoxylin and eosin (H&E) for routine histological examination to study the general structure.
Statistical analysis
All data were expressed as means ± SD and compared using the one-way ANOVA followed by Tukey’s post hoc test. Level of probability (P value) less than 0.05 is used as the criterion of significance. Statistical analysis was performed using the statistical software package GraphPad Prism®, Version 5.00 for Windows (California, USA).
Results
The statistical comparison between control and Alo (20 mg/kg/day) revealed no significant difference; therefore, all comparisons were referred to the control group.
Effect of Alo on liver function enzymes
Serum levels of ALT [F (3, 28) = 62.02, P ˂ 0.0001] and AST [F (3, 28) = 113.1, P ˂ 0.0001] were significantly increased upon CP treatment by 3.5 and 3.7 folds, respectively, as compared to the control group. These serum levels were drastically decreased upon Alo treatment by 2.4 and 2.1 folds, respectively, when compared to CP-treated group (Fig. 1).
Figure 1.
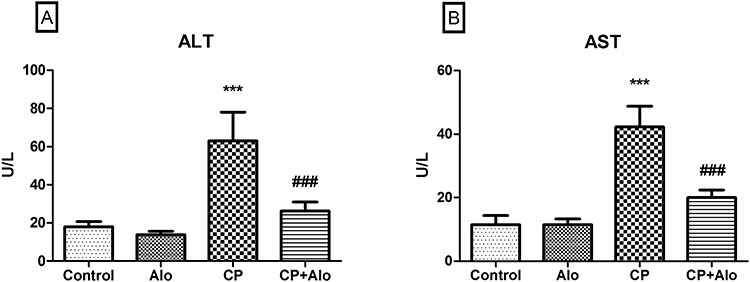
Effect of alogliptin (Alo) on serum (A) alanine transaminase (ALT), and (B) aspartate transaminase (AST) in cyclophosphamide (CP)-induced liver toxicity in rats. Data are presented as the mean ± SD (n = 8 per group; one-way ANOVA followed by Tukey’s multiple comparison test; ***P < 0.001, vs. the control group; ###P < 0.001, vs. the CP-treated group).
Effect of Alo on protein levels of hepatic phosphorylated PI3K (Tyr607), Akt (Thr450) and FoxO1 (Ser249)
Levels of p-PI3K (Tyr607) [F (3, 28) = 83.64, P ˂ 0.0001], p-Akt (Thr450) [F (3, 28) = 161, P ˂ 0.0001] and p-FoxO1 (Ser249) [F (3, 28) = 103.7, P ˂ 0.0001] were significantly decreased in CP-treated group by 5.7, 4.9 and 4.1 folds, respectively, as compared to the control group (Fig. 2). Following treatment with Alo, p-PI3K (Tyr607), p-Akt (Thr450) and p-FoxO1 (Ser249) protein levels were significantly elevated by 4.7, 3.5 and 3.1 folds, respectively, when compared to the CP-treated group.
Figure 2.
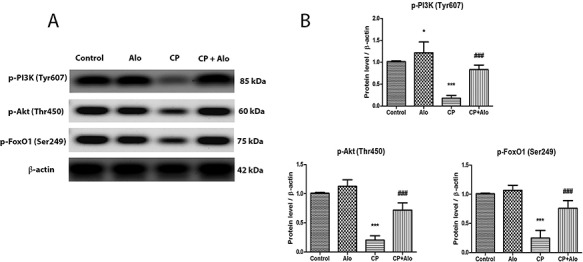
(A) Representative western blot bands of hepatic p-phosphoinositide 3–kinase (p-PI3K) (Tyr607), p-protein kinase B (p-Akt) (Thr450) and p-forkhead box transcription factor of the O class 1 (p-FoxO1) (Ser249) protein levels. (B) Quantitation of hepatic p-PI3K, p-Akt, and p-FoxO1 protein levels in control, alogliptin (Alo) (20 mg/kg/day; p.o.), cyclophosphamide (CP) (200 mg/kg single dose; i.p.) and CP + Alo-treated groups. Data are presented as the mean ± SD (n = 8 per group; one-way ANOVA followed by Tukey’s multiple comparison test; ***P < 0.001, vs. the control group; ###P < 0.001, vs. the CP-treated group).
Effect of Alo on hepatic SIRT1 expression, SOD and apoptotic markers levels
As shown in Fig. 3A–C, relative expression of SIRT1 [F (3, 28) = 31.26, P ˂ 0.0001], protein levels of the free radical scavenging enzyme SOD [F (3, 28) = 47.95, P ˂ 0.0001] and the anti-apoptotic marker Bcl-2 [F (3, 28) = 43.82, P ˂ 0.0001] were significantly repressed upon CP treatment by 3.6, 5.3 and 2.2 folds, respectively, when compared to the control group. Concurrently, the executioner pro-apoptotic enzyme caspase-3 levels [F (3, 28) = 154.8, P ˂ 0.0001] were significantly elevated upon CP treatment by 4.9 folds, as compared to the control group (Fig. 3D). Such effects were amended upon Alo treatment, where SIRT1 gene expression, SOD and Bcl-2 levels were significantly increased by 2.6, 3.6 and 1.9 folds, respectively; while caspase-3 levels significantly declined by 2.9 folds, when compared to the CP-treated group.
Figure 3.
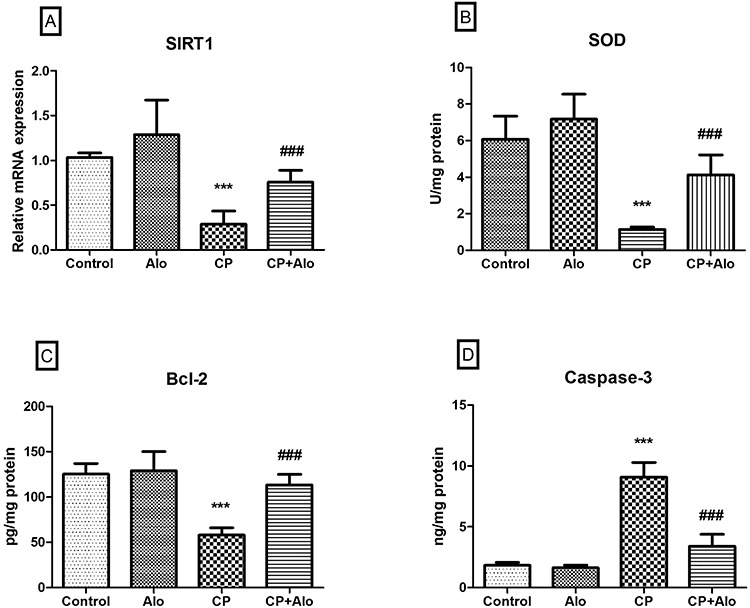
Effect of alogliptin (Alo) on gene expression of (A) sirtuin 1(SIRT1), and levels of (B) superoxide dismutase (SOD) and (C) B-cell lymphoma 2 (Bcl-2), and (D) caspase-3 activity in cyclophosphamide (CP)-induced liver toxicity in rats. Data are presented as the mean ± SD (n = 8 per group; one-way ANOVA followed by Tukey’s multiple comparison test; ***P < 0.001, vs. the control group; ###P < 0.001, vs. the CP-treated group).
Histopathological results of light microscopic examination of the liver sections
The photomicrographs of the H&E-stained liver sections (Fig. 4) of the control and Alo-treated groups show normal hepatocellular architecture depicted through normal hepatic cords arrangement around the central vein, separated by blood sinusoids, polyhedral eosinophilic granular hepatocytes with central rounded vesicular one or two nuclei.
Figure 4.
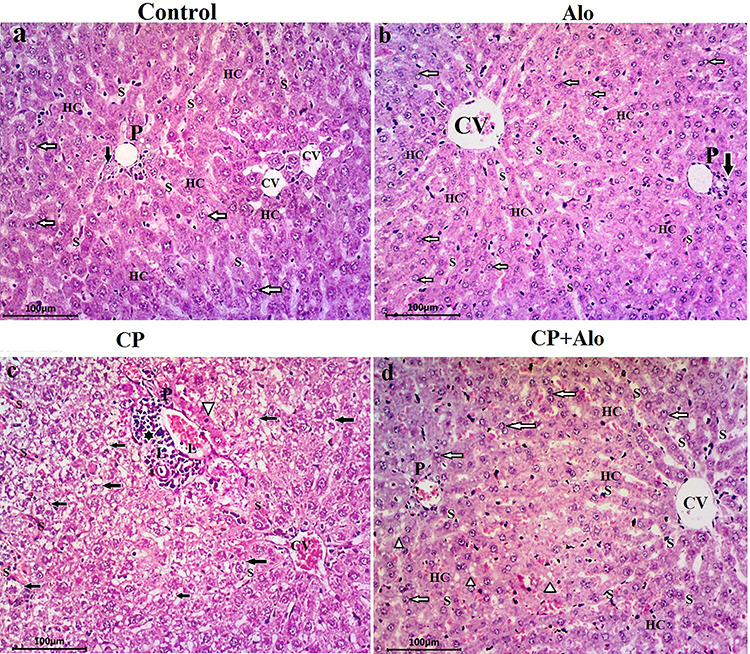
Photomicrographs of liver tissue sections. (a, b): Control and alogliptin (Alo)-treated groups show normal hepatocellular architecture portrayed through normal hepatic cords (HC) arrangement around the central vein (CV), separated by blood sinusoids (S), polyhedral eosinophilic granular hepatocytes with central rounded vesicular one or two nuclei (white arrows). The portal triad (P) at the corner of hepatic lobule shows a branch of hepatic artery, portal vein and bile duct (black arrow). (c): Cyclophosphamide (CP)-treated group displays disturbed hepatic lobular architecture. Congestion of both central (CV) and portal veins is observed. Marked mononuclear leukocyte infiltration (L) at the portal tract area (black star) and within the congested blood sinusoids (S) is noticed. Widespread vacuolated-ballooned hepatocytes in the form of microvacuolization of cytoplasm with centrally placed pyknotic nuclei (black arrows) are detected. Hepatocytes at portal tract area displayed apoptotic changes in form of dark eosinophilic cytoplasm and small deeply stained pyknotic nuclei (white arrowhead). (d): CP + Alo-treated group shows a potentially alleviated hepatocellular architecture compared to control group and minimal changes in the form of few vacuolated hepatocytes (white arrowhead) (H & E stain, X200).
Contrariwise, disturbed hepatic lobular architecture was observed in the liver architecture of the CP-treated group. This was shown in the congestion of central and portal veins, as well as the marked mononuclear leukocyte infiltration at the portal tract area and within the congested blood sinusoids. Furthermore, widespread vacuolated-ballooned hepatocytes in the form of microvacuolization of cytoplasm with centrally placed pyknotic nuclei are detected. Hepatocytes at portal tract area displayed apoptotic changes in the form of dark eosinophilic cytoplasm and small deeply stained pyknotic nuclei.
Remarkably, the CP + Alo-treated group showed a potentially alleviated hepatocellular architecture compared to control group and minimal changes in the form of few vacuolated hepatocytes.
Discussion
Several studies have previously investigated the deleterious effects imposed by CP upon various organs. However, to the best of our knowledge, our study reports, for the first time, the impact of Alo in alleviating CP-induced hepatotoxicity and the molecular mechanisms underlying this impact.
The hepatocellular damage in response to CP can be primarily attributed to the ROS produced in response to acrolein, the active metabolite of CP [29, 30]. One of the postulated mechanisms for acrolein-induced oxidative stress is attributed to the loss of glutathione [31]. This was supported in our study through the decreased levels of the anti-oxidant defenses, represented by SOD, which also came in agreement with the study of Shokrzadeh et al. in which CP-induced oxidative stress and its link to hepatotoxicity was revealed through reduced levels of SOD, catalase and reduced glutathione [32]. Similarly, these results came in accord with previous studies, in which cardiotoxicity and lung injury associated with decreased levels of anti-oxidant defenses were evident upon CP treatment under similar conditions to our study [33, 34].
The impact of oxidative stress imposed by CP was depicted in the reduced expression of SIRT1 as well as the decreased activity of PI3K and Akt signaling molecules. Scarce data are available regarding the change in SIRT1 expression as well as PI3K and Akt levels upon CP treatment. However, and in conjunction to our results, Xiang et al. supported our findings by reporting the importance of SIRT1 as a longevity protein and that its overexpression resulted in enhanced resistance to oxidative stress and apoptosis, and vice versa in SIRT1-deficient cells [35]. Similarly, previous studies have elaborated the involvement of deregulated PI3K/Akt pathway following doxorubicin chemotherapy and H2O2 treatment in mediating oxidative stress and subsequent apoptosis [36, 37].
The declined activity of SIRT1 and PI3K/Akt was further revealed in our study through the reduced phosphorylated levels of FoxO1. The reduced availability of p-FoxO1 suggests the increased nuclear levels and in turn gene expression of FoxO1 [12, 38]. Sequentially, FoxO1 up-regulates the downstream apoptotic genes and further contributes to cellular apoptosis [39]. Although previous studies have shown increased levels of SOD in response to increased FoxO1 gene expression [40], Manolopoulos et al. justified this as a short-term protective mechanism that ends up in favor of the more dominant oxidative stress and apoptosis [41]. This was supported by our current study through the decreased levels of the anti-apoptotic Bcl-2 following CP treatment, which was also revealed in previous studies [42]. As a result, and in consensus with aberrant SOD activity, the apoptotic cascade was activated as evident through increased levels of caspase-3 and perturbed hepatocellular architecture and liver function enzymes. This came in agreement with previous studies showing elevated hepatic function enzymes [32, 43], and disrupted histological picture manifested through lymphocyte infiltration, congestion of sinusoidal space, degeneration and necrosis of hepatocytes in the central region of liver tissue [24, 43], and at the cellular level activation of apoptosis through the intrinsic mitochondrial pathway, causing disturbance of the mitochondrial membrane potential and increased levels of executioner caspases [44].
Treatment with Alo showed improvement in multiple indices of hepatic injury, such as the liver function enzymes and the liver histopathological architecture. Tracking the molecular mechanisms behind Alo effect showed improved levels of the anti-oxidant SOD. This came in agreement with the study of Kabel, which showed improved anti-oxidant SOD levels following Alo treatment in doxorubicin-induced testicular atrophy [23]. This reduction in oxidative stress state was reflected in up-regulated SIRT1 expression. Concurrently, Alo treatment activated the PI3K/Akt signaling pathway. Such molecular changes mediated deacetylation of FoxO1 and inhibition of further gene transcription, allowing its translocation outside the nucleus and subsequent phosphorylation or inactivation. Limited data are available regarding the mechanistic effects of alogliptin; however, it was shown in the study of Lin and Huang that linagliptin, another DPP-IV inhibitor, was capable of activating SIRT1 and lowering oxidative stress through indirect increase of GLP-1 levels, in a similar mechanism of action to Alo [20].
Inactivation of FoxO1 allowed the anti-apoptotic Bcl-2 to modulate mitochondrial function and decrease cellular apoptosis, as witnessed through decreased levels of the executioner caspase-3, and the presence of more intact hepatocytes and vesicular nuclei in the histological sections. These findings can be supported by the study of Kabel in which caspase-3 and the apoptotic index were reduced following Alo treatment in doxorubicin-induced toxicity [23].
In conclusion, the current study succeeded to elaborate, for the first time, the promising effect of Alo in ameliorating chemotherapy-induced liver toxicity via tackling the SIRT1/FoxO1 and the PI3k/Akt pathways, resulting in abridged oxidative stress, apoptosis and hepatocellular injury.
Declarations of interest
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Abbreviations
Akt protein kinase B
Alo alogliptin
ALT alanine transaminase
AST aspartate transaminase
Bcl-2 B-cell lymphoma 2
CMC carboxymethyl cellulose
CP cyclophosphamide
DPP-IV dipeptidyl peptidase 4
FoxO forkhead box transcription factor of the O class
GLP-1 glucagon-like peptide 1
H&E Hematoxylin and Eosin
PI3K phosphoinositide 3–kinase
ROS reactive oxygen species
SIRT sirtuin
SOD superoxide dismutase
Contributor Information
Rania M Salama, Pharmacology & Toxicology Department, Faculty of Pharmacy, Misr International University (MIU), Cairo, Egypt; Translational and Clinical Research Unit, Faculty of Pharmacy, Misr International University (MIU), Cairo, Egypt.
Abdelkader M Mohamed, Translational and Clinical Research Unit, Faculty of Pharmacy, Misr International University (MIU), Cairo, Egypt.
Nada S Hamed, Translational and Clinical Research Unit, Faculty of Pharmacy, Misr International University (MIU), Cairo, Egypt.
Raneem M Ata, Translational and Clinical Research Unit, Faculty of Pharmacy, Misr International University (MIU), Cairo, Egypt.
Amira S NourelDeen, Translational and Clinical Research Unit, Faculty of Pharmacy, Misr International University (MIU), Cairo, Egypt.
Mohamed A Hassan, Translational and Clinical Research Unit, Faculty of Pharmacy, Misr International University (MIU), Cairo, Egypt.
References
- 1. Moignet A, Hasanali Z, Zambello R, et al. . Cyclophosphamide as a first-line therapy in LGL leukemia. Leukemia 2013;28:1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang D, Li L, Yang H, et al. . The constitutive androstane receptor is a novel therapeutic target facilitating cyclophosphamide-based treatment of hematopoietic malignancies. Blood 2013;121:329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bernstein JA, Garramone SM, Lower EG. Successful treatment of autoimmune chronic idiopathic urticaria with intravenous cyclophosphamide. Ann Allergy Asthma Immunol 2002;89:212–4. [DOI] [PubMed] [Google Scholar]
- 4. Starzl TE, Halgrimson CG, Penn I, et al. . Cyclophosphamide and human organ transplantation. Lancet 1971;2:70–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jonge ME, Huitema ADR, Beijnen JH, et al. . High exposures to bioactivated cyclophosphamide are related to the occurrence of veno-occlusive disease of the liver following high-dose chemotherapy. Br J Cancer 2006;94:1226–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mok CC, Wong WM, Shek TW, et al. . Cumulative hepatotoxicity induced by continuous low-dose cyclophosphamide therapy. Am J Gastroenterol 2000;95:845–6. [DOI] [PubMed] [Google Scholar]
- 7. Shokrzadeh M, Chabra A, Naghshvar F, Ahmadi A.. The mitigating effect of Citrullus colocynthis (L.) fruit extract against genotoxicity induced by cyclophosphamide in mice bone marrow cells. Sci World J 2013;2013:980480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Caito S, Rajendrasozhan S, Cook S, Chung S, Yao H, Friedman AE, et al. . SIRT1 is a redox-sensitive deacetylase that is post-translationally modified by oxidants and carbonyl stress. FASEB J 2010;24:3145–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cohen HY, Miller C, Bitterman KJ, et al. . Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 2004;305:390–2. [DOI] [PubMed] [Google Scholar]
- 10. Corbi G, Conti V, Russomanno G, Rengo G, Vitulli P, Ciccarelli AL, et al. . Is physical activity able to modify oxidative damage in cardiovascular aging? Oxid Med Cell Longev 2012;2012:728547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Duan W. Sirtuins: from metabolic regulation to brain aging. Front Aging Neurosci 2013;5:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sedding D, Haendeler J. Do we age on Sirt1 expression? Circ Res 2007;100:1396–8. [DOI] [PubMed] [Google Scholar]
- 13. Parcellier A, Tintignac LA, Zhuravleva E, et al. . PKB and the mitochondria: AKTing on apoptosis. Cell Signal 2008;20:21–30. [DOI] [PubMed] [Google Scholar]
- 14. Kops GJ, Ruiter ND, De Vries-Smits AM, et al. . Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature 1999;398:630–4. [DOI] [PubMed] [Google Scholar]
- 15. Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. . Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1999;96:857–68. [DOI] [PubMed] [Google Scholar]
- 16. Liu F, Huang X, Luo Z, et al. . Hypoxia-Activated PI3K/Akt Inhibits Oxidative Stress via the Regulation of Reactive Oxygen Species in Human Dental Pulp Cells. Oxid Med Cell Longev. 2019;2019:6595189. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17. Flatt PR, Bailey CJ, Green BD. Dipeptidyl peptidase IV (DPP IV) and related molecules in type 2 diabetes. Front Biosci. 2008;13:3648–60. [DOI] [PubMed] [Google Scholar]
- 18. Kim YO, Schuppan D. When GLP-1 hits the liver: a novel approach for insulin resistance and NASH. Am J Physiol Gastrointest Liver Physiol 2012;302:G759–61. [DOI] [PubMed] [Google Scholar]
- 19. Athauda D, Foltynie T.. The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson's disease: mechanisms of action. Drug Discov Today 2016;21:802–18. [DOI] [PubMed] [Google Scholar]
- 20. Lin CL, Huang CN. The neuroprotective effects of the anti-diabetic drug linagliptin against Abeta-induced neurotoxicity. Neural Regen Res 2016;11:236–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG, Group NCRRGW . Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 2010;160:1577–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Institute of Laboratory Animal Resources Guide for the Care and Use of Laboratory Animals: National Academy Press; 1996. 125 p. [Google Scholar]
- 23. Kabel AM. Zinc/alogliptin combination attenuates testicular toxicity induced by doxorubicin in rats: role of oxidative stress, apoptosis and TGF-beta1/NF-kappaB signaling. Biomed Pharmacother 2018;97:439–49. [DOI] [PubMed] [Google Scholar]
- 24. Caglayan C, Temel Y, Kandemir FM, et al. . Naringin protects against cyclophosphamide-induced hepatotoxicity and nephrotoxicity through modulation of oxidative stress, inflammation, apoptosis, autophagy, and DNA damage. Environ Sci Pollut Res Int 2018;25:20968–84. [DOI] [PubMed] [Google Scholar]
- 25. Koc A, Duru M, Ciralik H, et al. . Protective agent, erdosteine, against cisplatin-induced hepatic oxidant injury in rats. Mol Cell Biochem 2005;278:79–84. [DOI] [PubMed] [Google Scholar]
- 26. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 27. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54. [DOI] [PubMed] [Google Scholar]
- 28. Carson FL, Cappellano CH. Histotechnology. A self instructional text. 3rd ed Chicago: ASCP Press; 2009. [Google Scholar]
- 29. Ludeman SM. The chemistry of the metabolites of cyclophosphamide. Curr Pharm Des 1999;5:627–43. [PubMed] [Google Scholar]
- 30. Kern JC, Kehrer JP. Acrolein-induced cell death: a caspase-influenced decision between apoptosis and oncosis/necrosis. Chem Biol Interact 2002;139:79–95. [DOI] [PubMed] [Google Scholar]
- 31. Kehrer JP, Biswal SS. The molecular effects of acrolein. Toxicol Sci 2000;57:6–15. [DOI] [PubMed] [Google Scholar]
- 32. Shokrzadeh M, Chabra A, Ahmadi A, et al. . Hepatoprotective effects of zataria multiflora ethanolic extract on liver toxicity induced by cyclophosphamide in mice. Drug Res 2015;65:169–75. [DOI] [PubMed] [Google Scholar]
- 33. Nagi MN, Al-Shabanah OA, Hafez MM, et al. . Thymoquinone supplementation attenuates cyclophosphamide-induced cardiotoxicity in rats. J Biochem Mol Toxicol 2011;25:135–42. [DOI] [PubMed] [Google Scholar]
- 34. Shokrzadeh M, Ahmadi A, Chabra A, et al. . An ethanol extract of Origanum vulgare attenuates cyclophosphamide-induced pulmonary injury and oxidative lung damage in mice. Pharm Biol 2014;52:1229–36. [DOI] [PubMed] [Google Scholar]
- 35. Xiang Y, Xu J, Li L, et al. . Calorie restriction increases primordial follicle reserve in mature female chemotherapy-treated rats. Gene 2012;493:77–82. [DOI] [PubMed] [Google Scholar]
- 36. Kang KA, Wang ZH, Zhang R, et al. . Myricetin protects cells against oxidative stress-induced apoptosis via regulation of PI3K/Akt and MAPK signaling pathways. Int J Mol Sci 2010;11:4348–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sun X, Gu J, Chi M, et al. . Activation of PI3K-Akt through taurine is critical for propofol to protect rat cardiomyocytes from doxorubicin-induced toxicity. Can J Physiol Pharmacol 2014;92:155–61. [DOI] [PubMed] [Google Scholar]
- 38. Oellerich MF, Potente M. FOXOs and sirtuins in vascular growth, maintenance, and aging. Circ Res 2012;110:1238–51. [DOI] [PubMed] [Google Scholar]
- 39. Liu H, Xing R, Cheng X, et al. . De-novo NAD+ synthesis regulates SIRT1-FOXO1 apoptotic pathway in response to NQO1 substrates in lung cancer cells. Oncotarget 2016;7:62503–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci 2007;120(Pt 15):2479–87. [DOI] [PubMed] [Google Scholar]
- 41. Manolopoulos KN, Klotz LO, Korsten P, et al. . Linking Alzheimer's disease to insulin resistance: the FoxO response to oxidative stress. Mol Psychiatry 2010;15:1046–52. [DOI] [PubMed] [Google Scholar]
- 42. Abd El Tawab AM, Shahin NN, AbdelMohsen MM. Protective effect of Satureja montana extract on cyclophosphamide-induced testicular injury in rats. Chem Biol Interact 2014;224:196–205. [DOI] [PubMed] [Google Scholar]
- 43. Shokrzadeh M, Ahmadi A, Naghshvar F, et al. . Prophylactic efficacy of melatonin on cyclophosphamide-induced liver toxicity in mice. Biomed Res Int 2014;2014:470425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Strauss G, Westhoff MA, Fischer-Posovszky P, et al. . 4-hydroperoxy-cyclophosphamide mediates caspase-independent T-cell apoptosis involving oxidative stress-induced nuclear relocation of mitochondrial apoptogenic factors AIF and EndoG. Cell Death Differ. 2008;15:332–43. [DOI] [PubMed] [Google Scholar]


