Abstract
Since air pollutants are difficult and expensive to control, a strong scientific underpinning to policies is needed to guide mitigation aimed at reducing the current burden on public health. Much of the evidence concerning hazard identification and risk quantification related to air pollution comes from epidemiological studies. This must be reinforced with mechanistic confirmation to infer causality. In this review we focus on data generated from four contrasting sources of particulate air pollution that result in high population exposures and thus where there remains an unmet need to protect health: urban air pollution in developing megacities, household biomass combustion, wildfires and desert dust storms. Taking each in turn, appropriate measures to protect populations will involve advocating smart cities and addressing economic and behavioural barriers to sustained adoption of clean stoves and fuels. Like all natural hazards, wildfires and dust storms are a feature of the landscape that cannot be removed. However, many efforts from emission containment (land/fire management practices), exposure avoidance and identifying susceptible populations can be taken to prepare for air pollution episodes and ensure people are out of harm’s way when conditions are life-threatening. Communities residing in areas affected by unhealthy concentrations of any airborne particles will benefit from optimum communication via public awareness campaigns, designed to empower people to modify behaviour in a way that improves their health as well as the quality of the air they breathe.
Keywords: epidemiology, desert dust, household air pollution, megacities, particulate matter, toxicology, wildfires
Introduction
Particulate air pollution—a robust and consistent predictor of cardiorespiratory mortality and morbidity—originates from vehicle emissions, coal-burning power plants, industrial emissions and many other human-made as well as natural sources. As a consequence, the particle mix differs markedly—with respect to both type and quantity—in different parts of the world as a result of the pollutant source, population density and the weather. As expected therefore, estimations by the Global Burden of Disease (GBD) project of exposures to particulate matter less than 2.5 μm in diameter (PM2.5) amongst populations across the world from 1990 to 2017 display significant regional variation. Globally, there has been a slight decrease in the percentage of the world’s population residing in areas that exceed the air quality guideline (AQG; annual average PM2.5 concentration is set at 10 μg/m3) established by the World Health Organization (WHO), from 96% in 1990 to 92% in 2017 [1]. However these changes in air quality are unevenly distributed across different countries. Dramatic decreases have occurred in the USA, where the proportion of people living in areas exceeding the WHO guideline fell from 50% in 1990 to approximately 40% in 2010 and to only 3% in 2017. Other regions such as Japan and EU countries have also experienced improved air quality. Decreases in particulate air pollution over the past decades have yielded observable benefits to public health. For example, 13 years after the landmark Harvard Six Cities study highlighting the association between premature mortality and ambient PM2.5 [2], a re-evaluation of the situation showed that reductions in exposure contributed to significant and measurable improvements in life expectancy in the USA [3]. Furthermore, whilst the California Children’s Health Study showed that poor air quality was associated with slower growth in lung function of adolescents [4], they subsequently demonstrated that as air quality improved, so did lung growth [5].
Despite some success, substantial and long-standing challenges remain, and new ones have emerged. Of the latter, studies conducted in the USA, Canada and Europe have shown the reported associations between cardiorespiratory mortality/morbidity and PM2.5 concentrations below the WHO AQG [6–8], indicating that there is no safe lower limit for exposure. In addition, data suggest that airborne particle exposure may exert a wider threat to human health, beyond the cardiorespiratory systems, by negatively influencing a broader number of diseases including adverse birth outcomes [9–11], slower rates of cognitive development in children [12, 13] and accelerated cognitive decline in adults [14, 15]. Moreover, recent research utilising a hypothesis-free analysis suggests that many more new causes of hospital admission may be associated with short-term exposure to PM2.5 [16]. With respect to ongoing challenges, more than 90% of people worldwide still live in areas exceeding the WHO AQG, with less-developed countries suffering PM2.5 exposures that are 4 to 5 times higher than those of more-developed countries [1]. In addition, regions of the world are experiencing more frequent episodes of extreme air pollution brought about by natural phenomena.
Since air pollutants are difficult and expensive to control, a strong scientific underpinning to environmental health policies is needed to guide mitigation aimed at reducing ill health. Much of the evidence concerning hazard identification and risk quantification related to air pollution comes from epidemiological studies. This must be reinforced with mechanistic confirmation of the epidemiological findings to infer causality. This brief review focuses on data generated from both epidemiological and toxicological research on four contrasting sources of particulate air pollution that result in particularly high population exposures and thus where there remains an unmet need to develop appropriate policies and set effective mitigation strategies to assist in protecting human health: urban air pollution in rapidly developing megacities, biomass combustion in rural homes of developing countries, wildfires and desert dust storms.
Urban Air Pollution in Megacities of Developing Countries
The urban population of the world has grown rapidly from 751 million in 1950 to 4.2 billion in 2018 such that today, 55% of the world’s population lives in urban areas [17]. In some developing countries, the majority of urbanisation is linked to economic development. In Brazil and China, for example, the largest cities are concentrated in the largest economies. In other low-income regions, such as sub-Saharan Africa, mass movement to some cities occurs despite economic stagnation. Whilst urbanisation leads to social and economic progress from which residents can benefit from improved sanitation and access to health services, it also places enormous strain on infrastructure and ecosystems—not least owing to the addition of thousands of kilometres of urban roads and hundreds of millions of vehicles. The main environmental repercussion is the deterioration of ambient air quality and a recent investigation into the role that air pollution plays in the association of urbanisation with the global health showing that the penalty is stronger for low-income countries [18]. It is not surprising therefore that 24 of the 31 world’s megacities are located in less-developed countries that experience negative health impacts of poor air quality [19]. The burden is exceptionally high, multidimensional and complex in highly populated SE Asia, which is one of the fastest developing regions in the world with some of the highest urbanisation rates. Here, the low cost and domestic abundance of coal and some of the world’s worst traffic congestion is coupled with a widespread landscape fire challenge that causes some of the world’s worst pollution events. Owing to the increasing availability of air quality, epidemiological and toxicological data, this section will focus on megacities in China where rapid urbanisation and economic growth have been accompanied by significant increased emissions of air pollutants [20].
Effect on air quality
The “Campaign on Atmospheric Aerosol Research” network of China (CARE-China)—a project that includes 3 years of PM2.5 mass concentration observations (2012–2014)—reported an average PM2.5 concentration from 20 urban sites of 73.2 μg/m3 (16.8–126.9 μg/m3) [21]. The highest were observed at the urban stations of Xi’an (125.8 μg/m3), Taiyuan (111.5 μg/m3), Ji’nan (107.5 μg/m3) and Shijiazhuang (105.1 μg/m3), which are located in the most polluted areas of the Guanzhong Plain and the North China Plain. The averaged PM2.5 concentration in Beijing and Shanghai were approximately 70 and 56.2 μg/m3, respectively. Coal burning is the most important contributor to ambient PM2.5, responsible for 40% of population-weighted PM2.5 in China [22]. In recent years, however, China has moved aggressively to reduce air pollution beginning in 2013, with the Action Plan for Air Pollution Prevention and Control, which set key air quality targets and included specific actions to cut industrial emissions, reduce reliance on coal, control vehicle numbers in certain cities and increase low-emission energy sources. In 2017 when this act expired, a new 3-year plan that targets more cities was issued. An analysis of air quality in 74 Chinese cities recently found that annual average PM2.5 concentrations fell by one-third from 2013 to 2017 [1]. However, challenges remain, with the population-weighted annual concentration of PM2.5 in China still exceeding the WHO guideline.
Health effects
In 2017, the GBD estimated that approximately 852 000 deaths were attributable to PM2.5 exposures in China [1]. Populations residing in large cities are particularly at risk [23]. Chinese epidemiologic literature on the adverse effects of air pollution has grown substantially such that it now constitutes an important and growing component of the international literature. For example, multicity studies have documented the effects of short-term exposure to particulate air pollution (total suspended particulates [TSP]/particulate matter less than 10 μm in diameter [PM10]) on mortality and morbidity from cardiovascular and respiratory disease [24, 25], and cohort studies have reported links between long-term exposure and mortality [26–29]. Of interest, there has been no direct epidemiologic evidence on mortality risk from long-term exposure to PM2.5. Indeed after conversion from TSP/PM10 to PM2.5, the Chinese air pollution cohort studies generally report lower exposure–response coefficients of PM2.5 compared to studies in developed countries. Explanations for potentially smaller effect sizes in China compared with developed countries are multi-fold. In addition to a possible flatter exposure–response curve at higher concentrations, differences in composition and toxicity of PM, populations, study design/setting and exposure assessments all have the potential to affect risk per unit increase of ambient pollutants.
Human clinical/toxicological studies
The series of aggressive policies (limiting vehicular traffic on roads and emissions from industrial, power generation and commercial facilities) launched by the Chinese government to reduce local and regional emissions that affected air quality in the greater Beijing metropolitan area in the period leading up to and during the 2008 Beijing Olympic Games presented a quasi-experimental opportunity to measure pollutants and health outcomes before, during and after the 2008 Olympics [30]. Zhang et al. evaluated a representative group of pulmonary, systemic and urinary biomarkers in 125 healthy young adults. Improvements in air quality (60 and 13% reductions in gaseous pollutants and PM2.5, respectively) during the Olympic period were associated with changes in some biomarkers of several pathophysiologic pathways through which PM may exert its effects—coagulation in the circulation (soluble P-selectin and von Willebrand factor), inflammation in the airways (fractional exhaled nitric oxide, exhaled breath condensate hydrogen ion, nitrite and nitrate) and the activation of oxidative stress (urinary 8-hydroxy-2′-deoxyguanosine). Beijing’s pollution-control measures were expected not only to reduce the mass concentrations but also to change the composition of PM2.5, thus influencing the extent and mechanisms of its adverse health effects. To this end, an investigation of in vitro biological responses to PM2.5 collected before and during the Olympics revealed that toxicity was substantially dependent on its chemical components [31]. Cytotoxicity of murine alveolar macrophages at equal mass PM2.5 concentrations was notably reduced by control measures, and a significant association was found between viability and elemental zinc in PM2.5. Endotoxin content correlated with all of the eight detected cytokines/chemokines; elemental and organic carbon correlated with four; arsenic and chromium correlated with six and three, respectively; iron and barium showed associations with two; and nickel, magnesium, potassium and calcium showed associations with one.
Cardiorespiratory health effects due to high PM exposure have been observed in the winter and autumn coinciding with increased use of fuel for heating [32–35]. Other studies showed large effects in summer [32, 36]. To understand how seasonal variations as well as which fractions drive health effects elicited by oxidative stress, Pardo et al. exposed mice (intratracheal [IT]; 20 μg every other day for a total of 5 times) to aqueous and organic extracts from PM2.5 collected in Beijing [37]. The overall dose was reported as equivalent to 21 μg/m3 of PM in humans. Collected extracts of heating season (HS)-PM contained higher concentrations of metals and polyaromatic hydrocarbons (PAH) than that of non-HS extracts. In the lungs, the organic extracts increased lipid peroxidation and reduced protection mechanisms (reduced nuclear factor erythroid 2-related factor 2 [Nrf2] pathway and related phase II detoxifying genes). In the liver, exposure to organic extracts increased Nrf2 protection genes and elevated lipid peroxidation adducts to a greater degree, and this was accompanied by increased expression of the cytochrome P-450 family of genes (Cyp1a1 and Cyp2e) related to detoxification of PAHs.
Geographical as well as seasonal disparities of particle toxicity have also been investigated. Jin et al. quantified the component-specific contribution to in vitro cytotoxicity and reactive oxygen species (ROS) formation in BEAS-2b human bronchial epithelial cells triggered by wintertime PM2.5 from Beijing and Guangzhou, the biggest megacities in northern and southern China, respectively [38]. These cities possess distinct geographical and urban features and, consequently, starkly contrasting air pollution profiles [21]. Enhanced PM2.5 pollution of Beijing is not only due to the primary emissions from local sources but also stagnant weather with weak winds and relatively low boundary layer heights. In contrast, Guangzhou has fewer coal-based industries as well as more dispersive weather conditions. Exposure to PM2.5 samples from both Beijing and Guangzhou resulted in concentration-dependent cytotoxicity and ROS formation in BEAS-2b cells. The cytotoxic potency of Beijing PM2.5 nearly doubled that of Guangzhou PM2.5, whilst the oxidative stress potency of Beijing PM2.5 samples triples that of Guangzhou. The PM2.5 from Beijing also contained approximately 5 times higher concentrations of metals and PAHs per unit mass. These chemicals combined explained 38 and 24% of PM2.5-induced ROS in Beijing and Guangzhou, respectively, whilst more than 60% of the effects remained to be resolved in terms of contributing chemicals (Fig. 1). The significant contribution from coal combustion and vehicular emissions in Beijing PM2.5 was suggested as the major source disparities of toxicologically active PAHs between the two cities.
Figure 1.
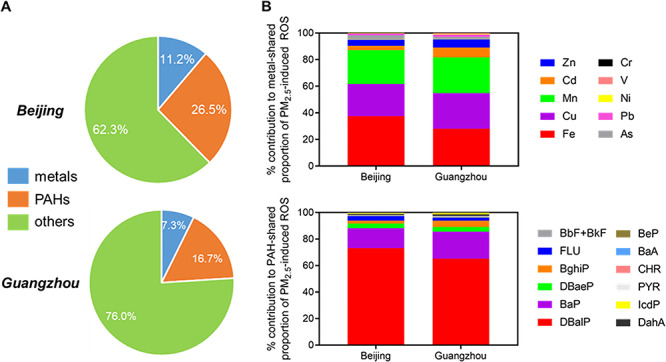
(A) Relative contribution of trace metals and PAHs to PM2.5-induced intracellular ROS in Beijing and Guangzhou and (B) individual chemical-resolved contributions to the metal- or PAH-shared ROS induction effects in Beijing and Guangzhou [38]. Data averaged from 14 and 11 samples for Beijing and Guangzhou, respectively. BaA, benzo(a)anthracene; BaP, benzo[a]pyrene; BbF, benzo[b]fluoranthene; BeP, benzo[e]pyrene; BghiP, benzo[g,h,i]perylene; BkF, benzo[k]fluoranthene; CHR, chrysene; DahA, dibenzene[a,h] anthracene; DBaeP, dibenzo[a,e]pyrene; DBalP, dibenzo[a,l]pyrene; FLU, fluoranthene; IcdP, indeno[1,2,3-cd]pyrene; PAH, polycyclic aromatic hydrocarbon; PYR, pyrene; ROS, reactive oxygen species. Reprinted with permission from (Jin L et al. Contributions of City-Specific Fine Particulate Matter (PM2.5) to Differential In Vitro Oxidative Stress and Toxicity Implications between Beijing and Guangzhou of China [38]. Environ Sci Technol 53(5):2881–2891. Copyright (2019) American Chemical Society
Household Air Pollution
In 2017, 47% of the world’s population (equating to 3.6 billion people) were exposed to a complex mixture of airborne particles and irritant gases as a consequence of burning biomass fuels (wood, charcoal, crop residues, animal dung) on inefficient stoves in poorly ventilated homes, for cooking, lighting and heating [1]. In addition to fine PM, biomass emissions contain carbon monoxide, nitrogen dioxide and a number of volatile and semi-volatile organic species depending on the type of fuel that is burned [39, 40]. The heterogeneous mix shares some characteristics with that of tobacco smoke, including its carcinogenic content. The greatest proportions of exposed populations live in low- and middle-income countries in Asia and Africa. Indeed, it is estimated that 846 million people in India (60% of the population) and 452 million people in China (32% of the population) were exposed to household air pollution (from the use of coal as well as biomass fuels in the case of China) in 2017 [1]. The magnitude of exposure, when one takes into account exposure intensity, time spent indoors and the number of individuals exposed, results in a far greater contribution of household air pollution (HAP) to global PM exposure than any other source [41].
Effects on air quality
Concentrations of particulate matter in homes where solid fuels are burned often exceed WHO ambient air quality guidelines by many orders of magnitude [42]. The inefficient combustion of solid fuels in poorly ventilated homes commonly creates mean 24-hour PM10 concentrations between 200 and 2000 μg/m3, whilst peak exposures of greater than 30 000 μg/m3 during periods of cooking have been reported [43]. In addition, the burning of solid fuels in and around the home is an important contributor to ambient air pollution and, as a consequence, to its disease burden so that populations relying on solid fuels bear a double burden. Recent global estimates suggest that residential energy use is responsible for 31% of global ambient PM2.5 concentrations [44] and the second largest sector (behind industry) in contribution to annual average ambient PM2.5-attributable mortality [22]. In China, of the total 916 000 deaths attributable to PM2.5 in 2013, about 19% of deaths (or about 177 000) were attributable to household burning of biomass and coal, which was more than from industrial or power plant combustion of coal [42].
Heath effects
Epidemiological studies conducted over the last few decades around the world have placed the residential burning of biomass amongst the most important global risk factors for early death and disease. In 2017, household air pollution contributed to 1.6 million deaths (2.9% of all deaths), with regional patterns reflecting population sizes and the proportion of each population using solid fuels. The greatest numbers of deaths were in India (482 000) and China (271 000) [1]. These countries accounted for about 46% of deaths attributable to household air pollution, whilst those in eastern, central and western sub-Saharan Africa, where 80 to 92% of the population relies on solid fuels, accounted for another 24% of deaths. Reviews by organisations such as the Health Effects Institute, the Institute of Health Metrics and Evaluation, the WHO and the International Agency for Research on Cancer (IARC) have concluded that the evidence points to a causal relationship between exposures to HAP and chronic obstructive pulmonary disease (COPD), acute lower respiratory infections, ischemic heart disease, stroke and cataracts [1]. In addition, the IARC has classified indoor burning of coal as a known human carcinogen and indoor burning of biomass as a probable human carcinogen [45]. The vulnerability of women and young children is a consequence of spending more time at home undertaking the cooking and heating and results in an incremental and ongoing (3–7 hours a day through a person’s lifetime) inhalation of small particles [46]. Assessments of respiratory morbidity amongst women cooking with biomass fuels compared to liquefied petroleum gas (LPG) show a doubled risk of airflow obstruction [47]. Moreover, in that different indices of lung function were significantly lower by 16–25 years suggests that biomass smoke during childhood may impair lung growth. In connection with such effects, a hypothesis links chronic PM exposure to suboptimal pulmonary development during childhood and an ensuing decline in lung function and increased vulnerability to COPD in adulthood in a chain of events involving oxidative stress, impaired innate immunity and subsequent bacterial infection [48].
Toxicological studies
It is perhaps not surprising therefore that the oxidative potential (the capacity of particulate pollution to cause damaging oxidative reactions, OP) of inhalable particles emitted during the burning of mixed biomass and wood has been demonstrated to be considerable and, in fact, greater than that of positive controls (National Institute of Standards and Technology and residual oil fly ash) [49, 50]. In another study, compared to woman who cooked with LPG, biomass fuel-using women exhibited airway and systemic inflammation and oxidative stress leading to a potentially adaptive cellular response consisting of Nrf2 activation and increased NAD(P)H:quinone oxidoreductase 1 (NQO1) protein concentrations in the airways [51].
The overall mechanisms by which solid fuel smoke causes adverse health effects are likely to be similar to those involved in tobacco smoke. Indeed a comparative analysis of dung biomass and cigarette smoke exposure in human small airway epithelial cells and mice (dung, 200 μg/m3 1 hour/day for 7 days; cigarette smoke, 80 μg/m3 6 hours/day for 7 days) demonstrated the activation of similar pathogenic processes, such as inflammation and protease expression, known to disrupt pulmonary structure [52]. In fact mice exposed to biomass exhibited more perivascular inflammation and had higher granulocyte and granulocyte-macrophage colony-stimulating factor (GM-CSF) lavage fluid levels than those exposed to cigarette smoke.
Two of the most prevalent biomass fuels are wood and cow dung, and in comparing relative toxicity, acute exposures (single 50 μg dose, nose aspiration) to mice resulted in pronounced neutrophilic inflammation, proinflammatory cytokine production, airway resistance and hyperresponsiveness, which were significantly higher in cow dung PM-exposed animals [53]. Subchronic exposures (50 μg × 3/week for 8 weeks, nose aspiration), however, induced eosinophilic inflammation, PM-specific antibody responses and alveolar destruction, which was greatest in wood PM-exposed mice. The potentially greater toxicity of cow dung biomass smoke, on the basis of oxidative capacity, particulates per mass of fuel burned and concentrations of microbial products, compared to other combustion products [49, 53, 54], has prompted an examination of six different types of dung biomass smoke on lung cells [55]. Since the type of animal dung used varies widely depending on local agro-geography, the types tested included horse (Equus caballus), US domestic cow (Bos taurus taurus), Indian domestic cow (Bos taurus indicus), African elephant (Loxodonta africana), goat (Capra hircus) and white rhinoceros (Ceratotherium simum). Dung biomass smoke, regardless of species, however, was reported to upregulate inflammatory mediators, activate pro-inflammatory transcription factors and attenuate innate immune mediator production in response to a viral mimetic in human airway epithelium cells.
Carbon loading of human alveolar macrophages has also been demonstrated in vivo in adults exposed to household air pollution and reproduced in vitro in cells exposed to respirable-sized carbon black particulates [56, 57]. These studies have not only demonstrated dose-related increases in cytokine production and decreases in phagocytosis and oxidative burst function in response to smoke exposure, but also that airway macrophage black carbon content is 36% lower in Malawian women using a cleaner burning biomass-fuelled cookstove compared with those using open fires for cooking and lower again healthy British women living in London who cooked with gas (Fig. 2).
Figure 2.
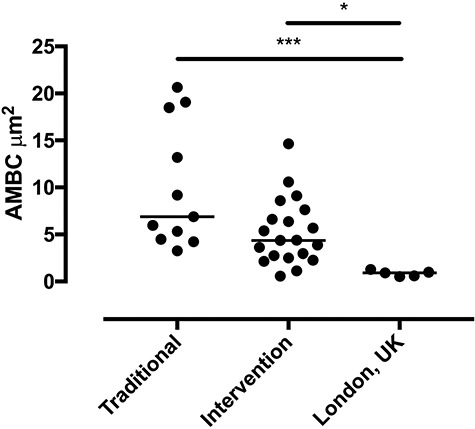
Comparison of airway macrophage black carbon (AMBC) results between the traditional cookstove group and the intervention cleaner cookstove group and the healthy UK controls, each dot represents a separate individuals mean AMBC (per 50 macrophages), P < 0.05 by Kruskall–Wallis and post hoc testing [56]
Wildfires
Wildfires are a global occurrence that temporarily produces a substantial amount of smoke pollution. This is a very complicated mixture that can come from lots of different fuel sources, including mature trees, dried leaves, forest litter and, unfortunately, local homes. The key primary emissions from wildland fires that worsen air quality and are of concern to public health include PM, carbon monoxide, oxides of nitrogen (NOx), ozone (O3), volatile organic compounds (VOCs), PAHs and benzene [58]. Of these, PM is the most common pollutant from wildfire smoke [59]. Measuring approximately 0.4 to 0.7 μm (the dominant aerodynamic diameter based on mass concentration distributions), it is also the principal pollutant of health concern from wildfire smoke. Air quality is further affected by the formation of secondary pollutants such as organic aerosols and O3 generated by the photoreaction of NOx and VOCs in the atmosphere [60–62]. The quantity, chemical composition, atmospheric transport and toxicity of wildfire smoke are influenced by many factors, including but not limited to type of fuel, phase of combustion, characteristics of the landscape, rate of fuel consumption, weather conditions and season [63, 64]. An increased wildfire activity (i.e. fires that burn more intensely, occur more frequently and can spread faster) can be at least partly attributed to climate change [65, 66]. In the western USA, large forest fires have been nearly five times as frequent as they were 50 years ago [67]. Furthermore, the first fires are starting earlier, and the last fires are starting later, extending the average wildfire season by about 75 days. In 2017 and 2018, California experienced the largest, most destructive and deadliest blazes recorded in the state’s long history of wildfires [68]. In Australia in recent years, large fires have also occurred more frequently and in more densely populated areas. These trends have been accompanied by a decline in average rainfall between April and May by 25% [69], whilst 9 out of Australia’s top 10 warmest years have occurred since 2005 [70].
Effect on air quality
Smoke from landscape fires can affect both the outdoor and indoor air quality of communities in rural and urban areas, sometimes hundreds of kilometres from the source [71]. The recent wildfires in south-east Australia have led to concentrations of ambient PM2.5 in Canberra and Sydney exceeding 200 μg/m3 and 400 μg/m3, respectively. Comparable concentrations have been reached in the past during other wildfire events and in other large Australian cities [72, 73]. Indoor filtration rates of outdoor PM2.5, such as those emitted from wildfires, are known to be higher compared to the coarse and ultrafine particulate matter size ranges (Liu et al. 2003; Liu et al. 2001; Long et al. 2001). A study measuring PM2.5 concentrations inside and outside 5 homes situated 11 to 47 kilometres away from 4 different landscape fires and one prescribed burn in Colorado reported outdoor 24-h average PM2.5 concentrations of 6–38 μg/m3. In comparison, 24-h average concentrations of 2–5 μg/m3 were measured at the same locations when no fires were burning [74]. During a distant Californian wildfire, particle number concentrations in a classroom in the San Francisco Bay Area were 10–15 times higher than those during non-wildfire days in the classroom [75].
Health effects
Of all wildfire-related air pollutants, investigations into the health effects of PM2.5 have created the most interest because of its known relationship to human health. Consistent evidence from a large number of studies indicates that wildfire smoke exposure is associated with all-cause mortality but no clear evidence for specific causes [76]. Consistent evidence documents associations between wildfire smoke exposure and general respiratory health effects (risk of hospitalisation, use of respiratory medication, cough, wheeze and eye irritation) and specifically, for exacerbations of asthma. There is less clear evidence of a link to cardiovascular outcomes [76–78]. The lack of consistency in findings could be due to difficulties in estimating how much PM people are exposed to during wildfire. These difficulties arise because of (a) insufficient monitoring data to capture how smoke plumes vary in space and time (when wildfire smoke moves through an area, it does not affect all portions of that area equally and the smoke plumes are usually not well mixed) and (b) high human mobility in the wake of evacuation.
Toxicological studies
Animal studies using mice have shown that wildfire PM collected in Northern California during the severe 2008 California fires was more toxic to the mouse lung on an equal dose basis (10 to 100 μg; IT) than PM collected from normal ambient air in the region [79–81]. Toxicity was manifested as increased numbers of macrophages/increased neutrophils and protein in lung lavage, histologic indicators of increased cell influx and pulmonary oedema [79], decreased antioxidant concentrations in the lung lavage fluid [80] and a greater increase in oxidative stress [81]. Studies in vitro using the RAW 264.7 macrophage cell line treated with the coarse fraction of wildfire PM, compared to coarse PM collected from the same region when wildfires were not occurring, demonstrated increased NF-kB expression, biomarkers of oxidative stress and cytotoxicity [82].
Experimental work has also studied the differential pulmonary and cardiovascular system effects in response to PM collected during the 2008 Pocosin Lakes National Wildlife Refuge fire in eastern North Carolina [83]. Particulate matter, obtained from the peat fire whilst smouldering or when nearly extinguished, was divided into ultrafine, fine and coarse fractions and administered (100 μg) to mice by oropharyngeal aspiration (OA). Coarse particle exposure elicited pro-inflammatory responses including increases in bronchoalveolar lavage fluid protein, cytokines (IL-6, TNF-α, MIP-2), neutrophils and intracellular ROS production. Exposure to fine or ultrafine particles did not produce pulmonary or systemic effects. In contrast, mice exposed to ultrafine PM developed significantly decreased cardiac function and greater post-ischemia-associated myocardial infarction. The same group of investigators has studied the cardiopulmonary effects in rats following exposure (35 or 350 μg, OA) to PM extracts collected from peat fire smoke. Responses included transient changes in ventilatory patterns within minutes of exposure and an altered regulation of left ventricular volume in the heart and pulmonary artery haemodynamics 1 day later [84].
In order to gain a greater understanding of the long-term health effects of wildfire smoke exposure in paediatric populations, Black et al. evaluated immunity and airway physiology (at approximately 3 years of age) in a cohort of outdoor-housed adolescent rhesus macaque monkeys that were exposed as infants (7–8 weeks of age) to ambient wood smoke from a series of Northern California wildfires in the summer of 2008 [85]. Wildfire-PM2.5 exposures above the National Ambient Air Quality Standards took place in two episodes consisting of 4–6 days each, peaking at 78 μg/m3. Modulation of peripheral blood Toll-like receptor (TLR)-induced cytokine synthesis (IL-6 and IL-8) was observed that was significantly associated with early-life wildfire smoke. Wildfire smoke-exposed monkeys also displayed significantly reduced inspiratory capacity, residual volume, vital capacity, functional residual capacity and total lung capacity per unit of body weight relative to control animals. In addition, there was a trend toward reduced total lung capacity in animals exposed to wildfire smoke as infants (Fig. 3).
Figure 3.
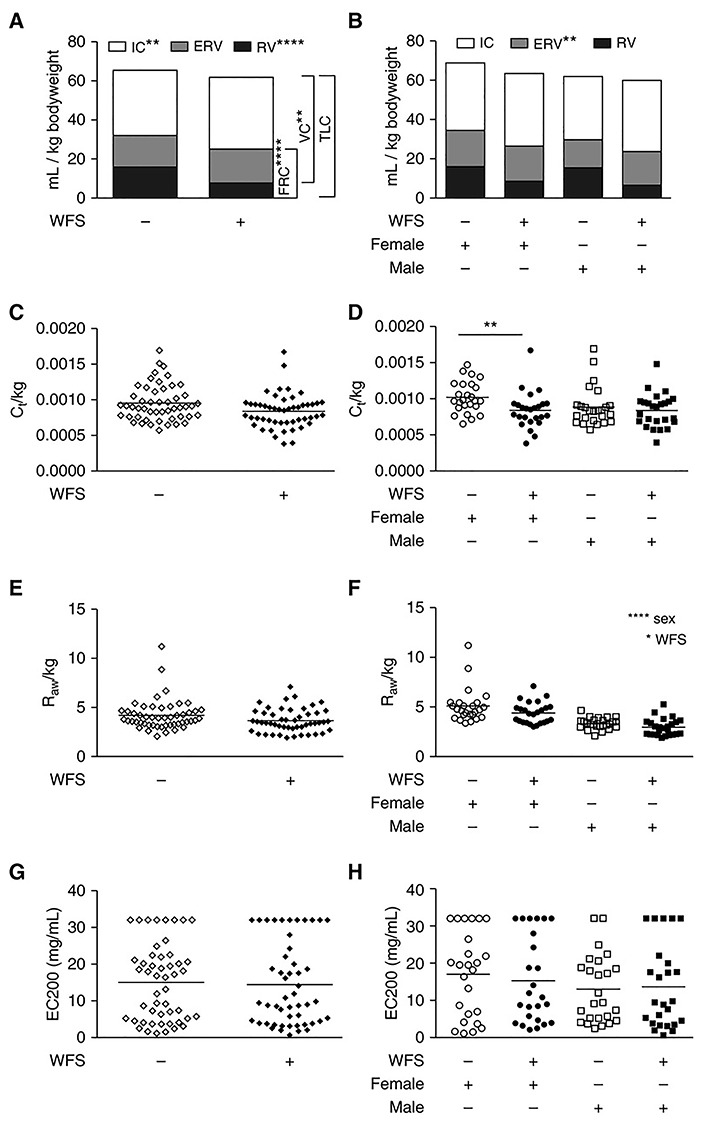
Effect of wildfire smoke exposure on lung volumes standardised to body weight in adolescent monkeys. **P < 0.01; ****P < 0.0001 [85]. IC, inspiratory capacity; RV, residual volume; TLC, total lung capacity; VC, vital capacity; WFS, wildfire smoke
In vivo studies in humans have also demonstrated increased inflammatory responses in military recruits exposed to an acute air pollution episode in Singapore during the 1997 south–east Asian smoke haze. The predominant pollutant during the haze period was PM10 that showed an increase of approximately 300% (haze 125.4 ± 44.9 versus post-haze 40 ± 14.3 μg/m3). Subjects developed leucocytosis that was associated with bone marrow stimulation [86] and elevated circulating concentrations of IL-1β, IL-6 and GM-CSF [87]. These responses were not accompanied by changes in lung function [86].
Desert Dust Storms
Desert dust is the mixture of PM emitted from the surface of arid and semi-arid areas around the world, with the Sahara and Sahel regions of North Africa being the most active dust sources emitting 790–840 million tons per year [88]. High winds raise large quantities of dust from arid surfaces, causing extremely high concentrations locally. Such a phenomenon is called a ‘dust storm’. Depending on weather conditions, desert dust can be transported near surface levels or lofted to high altitudes and from there can travel great distances. For example, Saharan dust is carried thousands of kilometres to the Americas [89], Europe [90], the Near East [91] and the Arctic [92]. Desert dust PM primarily constitutes mineral matter (silicon, aluminium, calcium, iron) but the size and specific composition of the dust changes with distance from source. For example, mass diameter measures 5–7 μm close to sources and 3–5 μm at receptor sites. It should be noted however that although the majority falls into the PM10 size, for high concentrations of dust, even if the proportion of PM2.5 in PM10 reaches only 5–35%, the PM2.5 absolute concentration might be very high as well. Throughout long-range transportation, desert dust combines with anthropogenic emissions of highly polluted industrial regions [93] as well as biological material, including pollens and microorganisms such as β-glucan and lipopolysaccharide (LPS), the major structural components of Gram-negative bacteria [94] and fungi [95], respectively. Dust derived from alkaline soil also captures acid gases such as sulphur (SOx) and nitrogen oxides (NOx) that are produced from fossil fuel combustion in industrialised areas. Once adsorbed onto particles of sand, sulphur dioxide (SO2) and nitrogen dioxide (NO2) subsequently form sulphates (SO42−) or nitrates (NO3−), respectively [96, 97]. In certain parts of the world, though not all, the frequency and scale of dust storms have increased in response to land use and climatic changes [98]. Current environmental fluctuations such as desertification and climate change indicate a future expansion of the global area of dry land [99] and an increase in the risk of drought [100].
Effect on air quality
Affected regions show increased ambient air dust concentrations that may last several days. In areas such as the southern Europe, Saharan dust events are a recurrent air quality problem [101], with PM10 and PM2.5 concentrations reaching very high concentrations during desert dust episodes, especially in the proximity of the source (>1000 μg/m3) areas but also at distant regions (up to 400–600 μg/m3) [88]. The measurement of dust storm pollution particles in an office building in Taipei during several sampling periods reported a 3-fold increase in the concentrations of PM2.5 and PM10 during dust storms and a high correlation between the indoor and outdoor air PM owing to ventilation systems in high-rise buildings utilising air from outside [102]. After adjusting for other covariates, autoregression models indicated that during dust storms, PM2.5 and PM10 in the indoor air increased significantly by 21.7 and 23.0 μg/m3.
Health effects
Desert dust storms have adverse health cardiorespiratory effects on a global scale owing to the transportation of dust particles over long distances [103]. A review by Karanasiou et al. on the health effects of Sahara dust episodes in Europe concluded that although there is no association of PM2.5 with total or cause specific daily mortality during Saharan dust intrusions, results for PM10 and PM2.5–10 were more inconsistent [104]. Some studies found no evidence of increased mortality due to PM10 during Sahara dust days, whilst other studies did report higher associations of PM10 and PM2.5–10 with mortality and morbidity on dust versus non-dust days [105–109]. A more recent review of the health impact from desert dust particles across the world reveals that both positive and negative associations have been reported for PM10 but only a positive relationship between PM2.5–10 and mortality [110]. This review also reported positive relationships between PM2.5 and mortality. Plausible reasons to explain these discrepancies are multi-fold: differences in the geographical distribution of dust source areas, study locations and dust trajectories. In addition, it is likely that the toxicological potential of the desert dust PM is modified upon mixing with anthropogenic emissions and biological components during different transport paths.
Toxicological studies
The toxicological literature contains a large number of studies that have investigated the respiratory effects of desert sand dust and in main, Asian sand dust (ASD) using doses that reflect or at least approach real-world exposures during a dust event. This experimental work has focused on both the virgin sand dust particles collected from surface soils (i.e. at the source) plus dust storm particles, that is, ‘windblown’ or ‘ambient’ sand dust sampled at a receptor site. Comparisons of toxicity between these types help to determine contributions from the dust particles themselves plus other possible ‘adhered’ responsible factors. Studies have demonstrated that single and repeated airway exposure of mice to ASD or ambient ASD (AASD) induces inflammatory lung injury in the lower respiratory tract [111–113] as well as exacerbating Klebsiella pneumoniae-induced pneumonia [114]. The aggravating effects, after IT instillation, on allergen (and particularly OVA)-induced lung eosinophilia have been extensively investigated in murine models of asthma and have demonstrated that this is orchestrated by cytokines, chemokines and antigen-specific immunoglobulin potentially via a TLR/MyD88 signalling pathways [115–118]. Exposure studies that have heated dust particles at 360°C to eliminate organic substances and chemicals [112, 115], sampled AASD of differing compositions [117, 119] or looked at the effects of added materials [112, 120–123] suggest these components may contribute to adverse health effects. Whilst the responsible factors have not been definitively defined, evidence points to PM-bound trace microbial elements and PAHs rather than sulphates. Furthermore, studies have demonstrated that the desert sand dust particle itself, rather than its constituents, can cause acute inflammatory changes and degeneration of the structure of the air–blood barrier [113, 124] plus that the aggravating effects are dependent on SiO2 content [125]. Together, these findings suggest that in addition to the involvement of adhered chemical and biological pollutants, mineralogical components are also candidate activators of immune and toxicological responses.
The relative toxicity of desert sand dust, compared to urban PM, has also been investigated. Comparisons with and composition analyses of urban PM2.5 suggest that the allergic inflammatory responses are greater in microbial element (β-glucan)-rich ASD-PM2.5 than in organic chemical-rich U-PM2.5 [126]. Aerosols generated during dust events do, however, seem to have a lower OP compared to combustion-generated PM sampled during non-dust periods [127, 128]. Other insightful findings from the in vitro literature are that the significant amounts of suspended desert sand dust during storm periods may provide a platform to intermix with chemicals on its surfaces, thereby increasing the bioreactivity of PM2.5 during dust storm episodes [129] and that mineral dust surface reactions are an unrecognised source of toxic organic chemicals in the atmosphere and that they enhance the toxicity of mineral dust aerosols in urban environments [130]. Atmospheric nitrated polycyclic aromatic hydrocarbons (NPAHs) have been shown to have adverse health effects such as carcinogenicity [131, 132]. They are produced in part through nitration reactions of parent PAHs in the atmosphere and subsequently deposit on airborne particulates. One of the most abundant NPAHs is 1-nitropyrene (1-NP), formed by the reaction of pyrene with NO2 on substrates including graphite and metal oxides [133, 134]. Working on the hypothesis that the heterogeneous formation of NPAHs on natural mineral dust could be particularly important owing to surface complexity and reactivity, the effects of heavy dust storms on ambient particle-associated 1-NP in Beijing have been examined [130]. Whilst kinetic experiments demonstrated that the reaction is accelerated on acidic surfaces of mineral dust, concentrations of ambient particle-associated NPAHs in Beijing were also found to significantly increase during heavy dust storms (Fig. 4).
Figure 4.
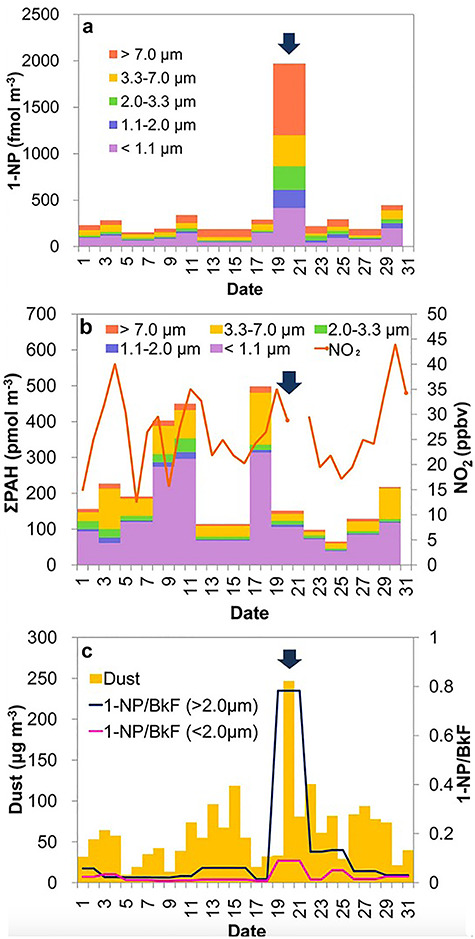
Atmospheric dust, PAHs, NO2 and 1-NP concentrations in Beijing in March 2010. (a) Size-fractionated particle-bound 1-NP. (b) Gaseous NO2 and size-fractionated particle-bound PAHs. (c) Aeolian dust. Variation in concentration of 1-NP relative to that of benzo[k]fluoranthene (BkF) (1-NP/BkF) is also shown in (c). Variation in concentration of 1-NP relative to that of a fairly unreactive and non-volatile PAH, BkF (1-NP/BkF) is also shown in (c). Arrows indicate a heavy dust period [130]. Arrows indicate a heavy dust period. 1-NP, 1-nitropyrene; BkF, benzo[k]fluoranthene; NO2, nitrogen dioxide; PAH, polycyclic aromatic hydrocarbons
Discussion
Looking forward, the human-made and natural sources of particulate air pollution discussed in this review will be influenced by a number of complex and overlapping variables that put simply can be categorised into further population growth/ urbanisation, climate change and air pollution mitigation measures. The world’s population is projected to reach almost 10 billion by 2050, and 68% of people will live in urban areas [17]. Nearly, 90% of this increase will take place in Asia and Africa. In predicting the repercussions on air quality and public health, estimations under hypothetical scenarios have been made. For example, under four different energy efficiency and air pollution-control scenarios, a GBD working group has projected the population-weighted mean exposure to PM2.5 in China to decrease (to 50, 38, 38 or 27 μg/m3) by 2030 [22]. Despite these projected reductions, the overall health burden is expected to increase as a consequence of an ageing population, susceptible to diseases most closely linked to air pollution. This particular exercise also calculated that even under the most stringent energy-use and pollution-control future scenario, coal would remain the single largest source contributor to ambient PM2.5 and its associated health burden in 2030. Solving the problem therefore constitutes a long-term goal for generations to come and requires more aggressive strategies to reduce emissions, particularly from coal combustion. Findings and recommendations such as these are strengthened by the output from the toxicological studies discussed herein. Pardo et al. showed that seasonal variations in pulmonary and hepatic toxicity of ambient PM2.5 in Beijing can be attributed to the increased coal burning during the cold winter months and the subsequent increases in PAH concentrations [37]. The study suggests that acute health effects of PM2.5 in this region of the world are stronger in the heating season and that the liver is a potentially susceptible organ to exposure to organic components such as PAHs. Given the incomplete understanding of differential particulate toxicity, the current global exercise in ascribing ill health to PM2.5 exposure relies on the assumption that particle toxicities are independent of composition [135]. The mixture toxicity modelling approach adopted by Jin et al. however adds to increasing evidence that points to the importance of understanding the contribution of associated components. The study revealed differential toxic mixtures of metals, PAHs plus unknown components occurring in PM2.5 that accounted for the varying oxidative effects elicited by PM2.5 from two megacities of China [38]. Whilst metals and PAHs were both found to be important contributing chemicals, metals may not be as dominant, and the relative importance of PAHs may also be site- and compound-specific. The average concentrations of PM2.5 in Beijing (220 ± 102 μg/m3) were approximately twice those of Guangzhou (104 ± 32 μg/m3) over the sampling period. However, should differential toxicities at an equal mass concentration be considered for city-specific scenarios, the exposure risks of PM2.5 in Beijing increase more than 4 times that in Guangzhou, thus highlighting the potential of developing integrated toxic indicators of relevance to specific health outcomes for effectively adjusting mass concentrations.
Climate change is forecasted to continue to affect the concentrations of both wildfire smoke and desert dust owing to increases in the scale and frequency of wildfires and dust storms, respectively, owing to shifts in temperatures, extended drought conditions, high winds and changes in vegetation. Climate models project that compared with the period 1961–1990, the mean area burned in California will increase by 77% by the end of this century [136]. Liu et al. estimated that PM2.5 exposures originating from wildfire smoke in the western USA for 2046–2051 under moderate climate change will be 160% higher than currently observed [137], whilst Ford et al. [138] projected that premature deaths attributable to fire-related PM2.5 will double by late twenty-first century compared to early twenty-first century under climate change scenarios. As mentioned earlier, PM2.5 concentrations are declining in most of the USA, but this is not the case in the Northwest USA where increasing concentrations are attributed to wildfires [71]. Likewise, a combination of environmental fluctuations such as desertification plus climate change indicates a future expansion of the global area of dry land, and with that, humans will be at an ever-increasing risk of frequent exposure to and the resultant adverse health effects of desert sand dust [99].
Existing toxicological evidence supporting potential respiratory and cardiovascular health effects of wildfire smoke exposure may help to resolve the current inconsistent epidemiological findings regarding associations with negative cardiovascular outcomes. For example, findings of Kim et al. suggested that cardiovascular effects might be mediated by the wildfire smoke’s fine fraction of PM whilst implicating the endotoxin content of coarse PM in pulmonary responses [83]. Furthermore, Thompson et al. presented evidence indicating that exposure to peat fire PM results in early and transient pulmonary irritation in conjunction with later alterations in heart function and thus strengthens the plausible linkage of air pollution exposure with early respiratory irritation and associated autonomic responses [84]. The study evaluating a cohort of outdoor-housed adolescent rhesus macaque monkeys exposed during infancy has important clinical implications, particularly in children, in supporting an association of wildfire smoke exposure during the postnatal period of development with immune dysregulation and compromised lung function in adolescence.
The toxicological studies on desert dust PM go some way in advising on respiratory health effects in that they suggest that atmospheric exposure to moulds and bacteria plus silica-carrying ASD may be a significant risk factor for inflammatory and allergic lung diseases such as child and adult asthma. In fact the large number of exacerbation studies may suggest that ASD-bound fungi, bacteria and silica-carrying particulate matter may turn asymptomatic or mild asthma into more severe cases. Moreover, the simultaneous exposure of asthmatic patients to ASD and its antigen may have serious consequences for such individuals. Other results appear to mimic cases reported by patients, suggesting pollen augments the influence of desert dust on asthmatic symptoms [139] and rhinoconjunctivitis [140]. Results also suggest that organic chemical components on/in ASDs may influence human respiratory health and that desert-PM2.5 may cause greater effects upon human respiratory health than organic chemical-rich urban-PM2.5.
Globally as economies have developed, there has been progress in the proportion of people cooking with solid fuels with a drop from about 57–47% between 2005 and 2017 [1]. During this time period in China, the proportion of households cooking with solid fuels fell from 61 to 32% (452 million) and in India from 76% in 2005 to 60% (846 million). However, the rates of solid fuel use and exposure to HAP remain high in less-developed countries, especially in eastern, central and western sub-Saharan Africa. In fact, despite declining usage rates in many countries of the world, the numbers of people potentially exposed may remain the same or even increase as populations continue to grow. Aggressive initiatives to reduce HAP exposure constitute a package of measures including the switching of fuel, improving cookstove efficiency, venting emissions and changing home layout and human behaviour [141, 142]. A proportion, but not all, of the studies assessing their effectiveness suggest that certain initiatives may reduce respiratory symptoms and lung function decline in women and severe pneumonia in children [143–145]. Factors that put forth to explain mixed effectiveness include low levels of adoption and/or sustained use and stove stacking where despite consistent use of clean stoves; households may continue to use polluting solid-fuel appliances for certain cooking tasks and the negative effects of neighbourhood sources of pollution (e.g. continued burning of solid fuels, rubbish and agriculture by nearby households). Despite these implementation challenges, the substantial exposures and health burdens from HAP mean the potential for delivering significant improvements via more strategic efforts.
Understanding the mechanisms underlying smoke-related health effects, such susceptibility to pulmonary infection and chronic lung disease, is not thoroughly understood, but such knowledge is key in providing biological evidence that public health interventions are needed to address the harm associated with the use of this fuel source. Toxicological studies are however making progress, characterising heightened inflammatory responses and impaired immune defences [52, 53, 55], and in doing so, suggest a mechanism by which HAP can directly cause lung diseases and promote increased susceptibility to infection. For example, in providing direct evidence that the use of cleaner burning cookstoves by women reduces alveolar macrophage black carbon loading, Whitehouse et al. suggests that in individuals who are regularly exposed to high levels of PM emitted from the burning of biomass, the use of a cleaner burning cookstove can reduce exposures which may in turn result in health benefits [56]. Whilst the nature and extent of such benefits are uncertain, the same workers reported that alveolar macrophage carbon particulate loading is inversely related to capacity to produce an effective antibacterial response [57] and thus speculated that reduced carbonaceous PM loading from the use of a cleaner cookstove reduces the risk of lower respiratory tract infection.
The exceptionally high concentration of particulate air pollution emanating from rapidly developing megacities, household biomass combustion, wildfires and dust storms calls for mitigating proposals, policies and measures, built on scientific knowledge. Taking each in turn will involve advocating smart cities, exploiting new transport technologies to create seamless end-to-end journeys that require fewer vehicles and promoting the transition from diesel and petrol engines to electric-powered and autonomous vehicles. The next steps to effectively act upon HAP should address economic and behavioural barriers to sustained adoption of clean stoves and fuels and other sources of combustion-related pollution in affected communities. Like all natural hazards, wildfires and dust storms are a feature of the landscape that cannot be removed. However, many efforts can be taken to prepare for air pollution episodes that arise in their wake and ensure people are out of harm’s way when conditions are life-threatening. Mitigation of the health effects caused by exposure to smoke includes management of emissions (land and fire management practices) as well as avoidance of exposure. Identifying communities vulnerable to adverse health outcomes from wildfire smoke and desert dust exposure can help prepare community-level responses, increase the community resilience and improve public health outcomes when episodes arise. Finally, communities residing in areas affected by any of the airborne particle sources addressed in this paper will benefit from optimum communication via public awareness campaigns designed to empower people to modify behaviour in a way that improves their health as well as the quality of the air they breathe.
Funding
This work was funded by the National Institute for Health Research (NIHR) Health Protection Research Unit in Environmental Exposures and Health, a partnership between Public Health England and Imperial College London. The views expressed are those of the author(s) and not necessarily those of the NIHR, Public Health England or the Department of Health and Social Care.
Conflict of interest statement
None declared.
References
- 1. Health Effects Institute. State of Global Air 2019. Special Report. Boston, MA: Health Effects Institute 2019 .
- 2. Dockery DW, Pope CA 3rd, Xu X et al. . An association between air pollution and mortality in six US cities. N Engl J Med 1993;329:1753–9. doi: 10.1056/nejm199312093292401. [DOI] [PubMed] [Google Scholar]
- 3. Pope CA 3rd, Ezzati M, Dockery DW. Fine-particulate air pollution and life expectancy in the United States. N Engl J Med 2009;360:376–86. doi: 10.1056/NEJMsa0805646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gauderman WJ, Vora H, McConnell R et al. . Effect of exposure to traffic on lung development from 10 to 18 years of age: a cohort study. Lancet 2007;369:571–7. doi: 10.1016/s0140-6736(07)60037-3. [DOI] [PubMed] [Google Scholar]
- 5. Gauderman WJ, Urman R, Avol E et al. . Association of improved air quality with lung development in children. N Engl J Med 2015;372:905–13. doi: 10.1056/NEJMoa1414123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shi L, Zanobetti A, Kloog I et al. . Low-concentration PM2.5 and mortality: estimating acute and chronic effects in a population-based study. Environ Health Perspect 2016;124: 46–52. doi: 10.1289/ehp.1409111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pinault LL, Weichenthal S, Crouse DL et al. . Associations between fine particulate matter and mortality in the 2001 Canadian census health and environment cohort. Environ Res 2017;159:406–15. doi: 10.1016/j.envres.2017.08.037. [DOI] [PubMed] [Google Scholar]
- 8. Khreis H, Cirach M, Mueller N et al. . Outdoor air pollution and the burden of childhood asthma across Europe. Eur Respir J 2019;54. doi: 10.1183/13993003.02194-2018. [DOI] [PubMed] [Google Scholar]
- 9. Klepac P, Locatelli I, Korosec S et al. . Ambient air pollution and pregnancy outcomes: a comprehensive review and identification of environmental public health challenges. Environ Res 2018;167:144–59. doi: 10.1016/j.envres.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 10. Stieb DM, Chen L, Eshoul M et al. . Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res 2012;117:100–11. doi: 10.1016/j.envres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 11. Li X, Huang S, Jiao A et al. . Association between ambient fine particulate matter and preterm birth or term low birth weight: an updated systematic review and meta-analysis. Environ Pollut 2017;227:596–605. doi: 10.1016/j.envpol.2017.03.055. [DOI] [PubMed] [Google Scholar]
- 12. Sunyer J, Esnaola M, Alvarez-Pedrerol M et al. . Association between traffic-related air pollution in schools and cognitive development in primary school children: a prospective cohort study. PLoS Med 2015;12:e1001792. doi: 10.1371/journal.pmed.1001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang X, Chen X, Zhang X. The impact of exposure to air pollution on cognitive performance. Proc Natl Acad Sci U S A 2018;115:9193–7. doi: 10.1073/pnas.1809474115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carey IM, Ross Anderson H, Atkinson RW et al. . Are noise and air pollution related to the incidence of dementia? A cohort study in London, England. BMJ Open 2018;8:e022404. doi: 10.1136/bmjopen-2018-022404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen H, Kwong JC, Copes R et al. . Exposure to ambient air pollution and the incidence of dementia: a population-based cohort study. Environ Int 2017;108:271–7. doi: 10.1016/j.envint.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 16. Wei Y, Wang Y, Di Q et al. . Short term exposure to fine particulate matter and hospital admission risks and costs in the Medicare population: time stratified, case crossover study. BMJ 2019;367:l6258. doi: 10.1136/bmj.l6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. United Nations World Urbanization Prospects. The 2018 Revision. New York: United Nations, 2019. [Google Scholar]
- 18. Wang Q. Urbanization and Global Health: the role of air pollution. Iran J Public Health 2018;47:1644–52. [PMC free article] [PubMed] [Google Scholar]
- 19. United Nations. The World’s Cities in 2016. New York: United Nations. 2016 . [Google Scholar]
- 20. He K, Huo H, Zhang Q. Urban air pollution in China: current status, characteristics, and progress. Annual Review of Energy and the Environment 2002;27:397–431. doi: 10.1146/annurev.energy.27.122001.083421. [DOI] [Google Scholar]
- 21. Liu Z, Goa W, Yu Y et al. . Characteristics of PM2.5 mass concentrations and chemical species in urban and background areas of China: emerging results from the CARE-China network. Atmospheric Chemistry and Physics 2018;18:8849–71. doi: 10.5194/acp-18-8849-2018. [DOI] [Google Scholar]
- 22. GBD MAPS Working Group Burden of Disease Attributable to Coal-Burning and Other Major Sources of Air Pollution in China. Special Report 20. Boston, MA: Health Effects Institute, 2016. [Google Scholar]
- 23. Maji KJ, Arora M, Dikshit AK. Burden of disease attributed to ambient PM2.5 and PM10 exposure in 190 cities in China. Environ Sci Pollut Res Int 2017;24:11559–72. doi: 10.1007/s11356-017-8575-7. [DOI] [PubMed] [Google Scholar]
- 24. Chen R., Haidong Kan, Bingheng Chen et al. Association of particulate air pollution with daily mortality: the China air pollution and health effects study. Am J Epidemiol 175, 1173–1181, doi: 10.1093/aje/kwr425 (2012). [DOI] [PubMed] [Google Scholar]
- 25. Wong CM, Vichit-Vadakan N, Kan H et al. . Public health and air pollution in Asia (PAPA): a multicity study of short-term effects of air pollution on mortality. Environ Health Perspect 2008;116:1195–202. doi: 10.1289/ehp.11257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cao J, Yang C, Li J et al. . Association between long-term exposure to outdoor air pollution and mortality in China: a cohort study. J Hazard Mater 2011;186:1594–600. doi: 10.1016/j.jhazmat.2010.12.036. [DOI] [PubMed] [Google Scholar]
- 27. Dong GH, Zhang P, Sun B et al. . Long-term exposure to ambient air pollution and respiratory disease mortality in Shenyang, China: a 12-year population-based retrospective cohort study. Respiration; International Review of Thoracic Diseases 2012;84:360–8. doi: 10.1159/000332930. [DOI] [PubMed] [Google Scholar]
- 28. Zhang P, Dong G, Sun B et al. . Long-term exposure to ambient air pollution and mortality due to cardiovascular disease and cerebrovascular disease in Shenyang, China. PLoS One 2011;6:e20827. doi: 10.1371/journal.pone.0020827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou M, Liu Y. Lijun Wang et al. particulate air pollution and mortality in a cohort of Chinese men. Environ Pollut 2014;186:1–6. doi: 10.1016/j.envpol.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 30. Zhang J, Zhu T, Kipen H et al. . Cardiorespiratory Biomarker Responses in Healthy Young Adults to Drastic Air Quality Changes Surrounding the 2008 Beijing Olympics. Boston, MA: Health Effects Institute, 2013. [PMC free article] [PubMed] [Google Scholar]
- 31. Shang Y, Zhu T, Lenz A-G et al. . Reduced in vitro toxicity of fine particulate matter collected during the 2008 summer Olympic games in Beijing: the roles of chemical and biological components. Toxicol In Vitro 2013;27:2084–93. doi: 10.1016/j.tiv.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 32. Chen R., Peng Roger D., Meng Xia et al. . Seasonal variation in the acute effect of particulate air pollution on mortality in the China Air Pollution and Health Effects Study (CAPES). Sci Total Environ 2013;450-451:259–65, doi: 10.1016/j.scitotenv.2013.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen Y. C., Chiang Hung-Che, Hsu Chin-Yu et al. . Ambient PM2.5-bound polycyclic aromatic hydrocarbons (PAHs) in Changhua County, Central Taiwan: seasonal variation, source apportionment and cancer risk assessment. Environ Pollut 2016;218:372–82, doi: 10.1016/j.envpol.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 34. Lu H, Wang S, Li Y et al. . Seasonal variations and source apportionment of atmospheric PM2.5-bound polycyclic aromatic hydrocarbons in a mixed multi-function area of Hangzhou. China Environ Sci Pollut Res Int 2017;24:16195–205. doi: 10.1007/s11356-017-9265-1. [DOI] [PubMed] [Google Scholar]
- 35. Qi L, Chen M, Ge X et al. . Seasonal variations and sources of 17 aerosol metal elements in suburban Nanjing, China. Atmos 2016;7:153. doi: 10.3390/atmos7120153. [DOI] [Google Scholar]
- 36. Ma Y, Chen R, Pan G et al. . Fine particulate air pollution and daily mortality in Shenyang, China. Sci Total Environ 2011;409:2473–7. doi: 10.1016/j.scitotenv.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 37. Pardo M, Xu F, Qiu X et al. . Seasonal variations in fine particle composition from Beijing prompt oxidative stress response in mouse lung and liver. Sci Total Environ 2018;626:147–55. doi: 10.1016/j.scitotenv.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 38. Jin L, Xie J, Wong CKC et al. . Contributions of City-specific fine particulate matter (PM2.5) to differential in vitro oxidative stress and toxicity implications between Beijing and Guangzhou of China. Environ Sci Technol 2019;53:2881–91. doi: 10.1021/acs.est.9b00449. [DOI] [PubMed] [Google Scholar]
- 39. Venkataraman C, Rao GUM. Emission factors of carbon monoxide and size-resolved aerosols from biofuel combustion. Environ Sci Technol 2001;35:2100–7. [DOI] [PubMed] [Google Scholar]
- 40. Raiyani CV, Jani JP, Desai NM et al. . Assessment of indoor exposure to polycyclic aromatic hydrocarbons for urban poor using various types of cooking fuels. Bull Environ Contam Toxicol 1993;50:757–63. [DOI] [PubMed] [Google Scholar]
- 41. Smith KR. Fuel combustion air pollution. Exposure and health: the situation in developing countries. Annu Rev Energy Environ 1993;18:529–66. [Google Scholar]
- 42. HEI Household Air Pollution Working Group Household air pollution and noncommunicable disease Communication, Vol. 18 Boston, MA: Health Effects Institute, 2018. [Google Scholar]
- 43. Balakrishnan K, Ghosh S, Ganguli B et al. . State and national household concentrations of PM2.5 from solid cookfuel use: results from measurements and modeling in India for estimation of the global burden of disease. Environ Health 2013;12:77. doi: 10.1186/1476-069x-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lelieveld J, Evans JS, Fnais M et al. . The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015;525:367–71. doi: 10.1038/nature15371. [DOI] [PubMed] [Google Scholar]
- 45. International Agency for research on Cancer Air Pollution and Cancer. Lyon, France: World Health Organization, 2013. [Google Scholar]
- 46. United Nations Millennium Project Investing in Development. A Practical Plan to Achieve the Millennium Development Goals. New York, USA: Millennium Project, 2005. [Google Scholar]
- 47. Kurmi OP, Devereux GS, Cairns S Smith W et al. . Reduced lung function due to biomass smoke exposure in young adults in rural Nepal. Eur Respir J 2013;41:25–30. doi: 10.1183/09031936.00220511. [DOI] [PubMed] [Google Scholar]
- 48. Grigg J. Particulate matter exposure in children: relevance to chronic obstructive pulmonary disease. Proc Am Thorac Soc 2009;6:564–9. doi: 10.1513/pats.200905-026RM. [DOI] [PubMed] [Google Scholar]
- 49. Mudway IS, Duggan ST, Venkataraman C et al. . Combustion of dried animal dung as biofuel results in the generation of highly redox active fine particulates. Part Fibre Toxicol 2005;2:6. doi: 10.1186/1743-8977-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kurmi OP, Dunster C, Ayres JG et al. . Oxidative potential of smoke from burning wood and mixed biomass fuels. Free Radic Res 2013;47:829–35. doi: 10.3109/10715762.2013.832831. [DOI] [PubMed] [Google Scholar]
- 51. Mondal NK, Saha H, Mukherjee B et al. . Inflammation, oxidative stress, and higher expression levels of Nrf2 and NQO1 proteins in the airways of women chronically exposed to biomass fuel smoke. Mol Cell Biochem 2018;447:63–76. doi: 10.1007/s11010-018-3293-0. [DOI] [PubMed] [Google Scholar]
- 52. Mehra D, Geraghty PM, Hardigan AA et al. . A comparison of the inflammatory and proteolytic effects of dung biomass and cigarette smoke exposure in the lung. PLoS One 2012;7:e52889. doi: 10.1371/journal.pone.0052889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sussan TE, et al. Source of biomass cooking fuel determines pulmonary response to household air pollution. Am J Respir Cell Mol Biol 2014;50:538–48. doi: 10.1165/rcmb.2013-0201OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gordon SB, Bruce NG, Grigg J et al. . Respiratory risks from household air pollution in low and middle income countries. Lancet Respir Med 2014;2:823–60. doi: 10.1016/s2213-2600(14)70168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McCarthy CE, Duffney PF, Wyatt JD et al. . Comparison of in vitro toxicological effects of biomass smoke from different sources of animal dung. Toxicol In Vitro 2017;43:76–86. doi: 10.1016/j.tiv.2017.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Whitehouse AL, Miyashita L, Liu NM et al. . Use of cleaner-burning biomass stoves and airway macrophage black carbon in Malawian women. Sci Total Environ 2018;635:405–11. doi: 10.1016/j.scitotenv.2018.04.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rylance J, Fullerton DG, Scriven J et al. . Household air pollution causes dose-dependent inflammation and altered phagocytosis in human macrophages. Am J Respir Cell Mol Biol 2015;52:584–93. doi: 10.1165/rcmb.2014-0188OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Naeher LP, Brauer M, Lipsett M et al. . Woodsmoke health effects: a review. Inhal Toxicol 2007;19:67–106. doi: 10.1080/08958370600985875. [DOI] [PubMed] [Google Scholar]
- 59. Jaffe D, Hafner W, Chand D et al. . Interannual variations in PM2.5 due to wildfires in the western United States. Environ Sci Technol 2008;42:2812–8. doi: 10.1021/es702755v. [DOI] [PubMed] [Google Scholar]
- 60. Phoothiwut S, Junyapoon S. Size distribution of atmospheric particulates and particulate-bound polycyclic aromatic hydrocarbons and characteristics of PAHs during haze period in Lampang Province, Northern Thailand. Air Quality, Atmosphere & Health 2013;6:397–405. doi: 10.1007/s11869-012-0194-3. [DOI] [Google Scholar]
- 61. Vicente A, Alves C, Calvo AI et al. . Emission factors and detailed chemical composition of smoke particles from the 2010 wildfire season. Atmos Environ 2013;71:295–303. doi: 10.1016/j.atmosenv.2013.01.062. [DOI] [Google Scholar]
- 62. Jaffe DA, Wigder NL. Ozone production from wildfires: a critical review. Atmos Environ 2012;51:1–10. doi: 10.1016/j.atmosenv.2011.11.063. [DOI] [Google Scholar]
- 63. Black C, Tesfaigzi Y, Bassein JA et al. . A. Wildfire smoke exposure and human health: significant gaps in research for a growing public health issue. Environ Toxicol Pharmacol 2017;55:186–95. doi: 10.1016/j.etap.2017.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kim YH, Warren SH, Krantz QT et al. . Mutagenicity and lung toxicity of smoldering vs. flaming emissions from various biomass fuels: implications for health effects from wildland fires. Environ Health Perspect 2018;126:017011. doi: 10.1289/EHP2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Abatzoglou JT, Williams AP. Impact of anthropogenic climate change on wildfire across western US forests. Proc Natl Acad Sci U S A 2016;113:11770–5. doi: 10.1073/pnas.1607171113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schoennagel T, Balch JK, Brenkert-Smith H et al. . Adapt to more wildfire in western north American forests as climate changes. Proc Natl Acad Sci U S A 2017;114:4582–90. doi: 10.1073/pnas.1617464114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Balmes J. Where there is wildfire there is smoke. N Engl J Med 2018;378:881–3. doi: 10.1056/NEJMp1716846. [DOI] [PubMed] [Google Scholar]
- 68. Reid CE, Considine EM, Watson GL et al. . Associations between respiratory health and ozone and fine particulate matter during a wildfire event. Environ Int 2019;129:291–8. doi: 10.1016/j.envint.2019.04.033. [DOI] [PubMed] [Google Scholar]
- 69.Climate Council Australia. This Is Not Normal: Climate Change and Escalating Bushfire Risk. https://www.climatecouncil.org.au/resources/new-report-this-is-not-normal-climate-change-and-escalating-bushfire-risk/ (12 June 2020, date last accessed). [Google Scholar]
- 70.Australian Government Bureau of Meteorology. Annual Climate Statement. 2019. http://www.bom.gov.au/climate/current/annual/aus/ (12 June 2020, date last accessed).
- 71. McClure CD, Jaffe DA. US particulate matter air quality improves except in wildfire-prone areas. Proc Natl Acad Sci U S A 2018;115:7901–6. doi: 10.1073/pnas.1804353115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. He C, Miljevic B, Crilley LR et al. . Characterisation of the impact of open biomass burning on urban air quality in Brisbane, Australia. Environ Int 2016;91:230–42. doi: 10.1016/j.envint.2016.02.030. [DOI] [PubMed] [Google Scholar]
- 73. Keywood M, Cope M, Meyer CPM et al. . When smoke comes to town: the impact of biomass burning smoke on air quality. Atmos Environ 2015;121:13–21. doi: 10.1016/j.atmosenv.2015.03.050. [DOI] [Google Scholar]
- 74. Henderson DE, Milford JB, Miller SL. Prescribed burns and wildfires in Colorado: impacts of mitigation measures on indoor air particulate matter. J Air Waste Manag Assoc 2005;55:1516–26. [DOI] [PubMed] [Google Scholar]
- 75. Kaduwela AP, Kaduwela AP, Jrad E et al. . Development of a low-cost air sensor package and indoor air quality monitoring in a California middle school: detection of a distant wildfire. J Air Waste Manag Assoc 2019;69:1015–22. doi: 10.1080/10962247.2019.1629362. [DOI] [PubMed] [Google Scholar]
- 76. Reid CE, Brauer M, Johnston FH et al. . Critical review of health impacts of wildfire smoke exposure. Environ Health Perspect 2016;124:1334–43. doi: 10.1289/ehp.1409277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Reid CE, Maestas MM. Wildfire smoke exposure under climate change: impact on respiratory health of affected communities. Curr Opin Pulm Med 2019;25:179–87. doi: 10.1097/mcp.0000000000000552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Liu JC, Pereira G, Uhl SA et al. . A systematic review of the physical health impacts from non-occupational exposure to wildfire smoke. Environ Res 2015;136:120–32. doi: 10.1016/j.envres.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wegesser TC. California wildfires of 2008: coarse and fine particulate matter toxicity. Environ Health Perspect 2009;115:893–7doi: 10.1289/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wegesser TC, Franzi LM, Mitloehner FM et al. . Lung antioxidant and cytokine responses to coarse and fine particulate matter from the great California wildfires of 2008. Inhal Toxicol 2010;22:561–70. doi: 10.3109/08958370903571849. [DOI] [PubMed] [Google Scholar]
- 81. Williams KM, Franzi LM, Last JA. Cell-specific oxidative stress and cytotoxicity after wildfire coarse particulate matter instillation into mouse lung. Toxicol Appl Pharmacol 2013;266:48–55. doi: 10.1016/j.taap.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Franzi LM, Bratt JM, Williams KM et al. . Why is particulate matter produced by wildfires toxic to lung macrophages? Toxicol Appl Pharmacol 2011;257:182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kim A, Tong H, Daniels M et al. . Cardiopulmonary toxicity of peat wildfire particulate matter and the predictive utility of precision cut lung slices. Part Fibre Toxicol 2014;11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Thompson LC, Kim YH, Martin BL et al. . Pulmonary exposure to peat smoke extracts in rats decreases expiratory time and increases left heart end systolic volume. Inhal Toxicol 2018;30:439–47. doi: 10.1080/08958378.2018.1551443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Black C, Gerriets JE, Fontaine JH et al. . Early life wildfire smoke exposure is associated with immune dysregulation and lung function decrements in adolescence. Am J Respir Cell Mol Biol 2017;56:657–66. doi: 10.1165/rcmb.2016-0380OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tan, WC, Qiu D, Liam BL et al. The Human Bone Marrow Response to Acute Air Pollution Caused by Forest Fires. Am J Respir Crit Care Med 2000;161:1213–17. [DOI] [PubMed] [Google Scholar]
- 87. Eeden SF, Tan WC, Suwa T et al. . Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10)). Am J Respir Crit Care Med 2001;164:826–30. doi: 10.1164/ajrccm.164.5.2010160. [DOI] [PubMed] [Google Scholar]
- 88. Querol X, Tobías A, Perez N et al. . Monitoring the impact of desert dust outbreaks for air quality for health studies. Environ Int 2019;130:104867. doi: 10.1016/j.envint.2019.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ben-Ami Y, Koren I, Rudich Y et al. . Transport of Saharan dust from the Bodélé depression to the Amazon Basin: a case study. Atmos Chem Phys Disc 2010;10:4345–72. [Google Scholar]
- 90. Varga G, Kovács J, Ujvári G. Analysis of Saharan dust intrusions into the Carpathian Basin (Central Europe) over the period 1979–2011. Global Planet Change 2013;100:333–42. [Google Scholar]
- 91. Thevenon F, Chiaradia M, Adatte T et al. . Ancient versus modern mineral dust transported to high-altitude alpine glaciers evidences Saharan sources and atmospheric circulation changes. Atmos Chem Phys Disc 2011;11:859–84. [Google Scholar]
- 92. Barkan J, Alpert P. Synoptic analysis of a rare event of Saharan dust reaching the Arctic region. Weather 2010;65:208–11. [Google Scholar]
- 93. Tamamura S, Okada Y, Tang N et al. . Long-range transport of polycyclic aromatic hydrocarbons (PAHs) from the eastern Asian continent to Kanazawa, Japan with Asian dust. Atmos Environ 2007;41:2580–93. doi: 10.1016/j.atmosenv.2006.11.021. [DOI] [Google Scholar]
- 94. Nikaido H. Structure of cell wall lipopolysaccharide from salmonella typhimurium. I. Linkage between o side chains and R core. J Biol Chem 1969;244:2835–45. [PubMed] [Google Scholar]
- 95. Hearn VM, Sietsma JH. Chemical and immunological analysis of the aspergillus fumigatus cell wall. Microbiology 1994;140:789–95. [DOI] [PubMed] [Google Scholar]
- 96. Mori I, Nishikawa M, Tanimura T et al. . Change in size distribution and chemical composition of Kosa (Asian dust) aerosol during long-range transport. Atmos Environ 2003;37:4253–63. [Google Scholar]
- 97. Sorimachi A, Sakai M, Ishitani O et al. . Study on dry deposition of SO2–NOx onto loess. Water Air Soil Pollut 2001;130:541–6. [Google Scholar]
- 98. Goudie AS. Desert dust and human health disorders. Environ Int 2014;63:101–13. doi: 10.1016/j.envint.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 99. Huang J, Yu H, Guan X et al. . Accelerated dryland expansion under climate change. Nature Climate Change 2015;6:166–71. doi: 10.1038/nclimate2837. [DOI] [Google Scholar]
- 100. Dai A. Increasing drought under global warming in observations and models. Nature Climate Change 2012;3:52–8. doi: 10.1038/nclimate1633. [DOI] [Google Scholar]
- 101. Rodriguez S, Querol X, Alastuey A et al. . Events affecting levels and seasonal evolution of airborne particulate matter concentrations in the western Mediterranean. Environ Sci Technol 2003;37:216–22. [DOI] [PubMed] [Google Scholar]
- 102. Kuo H-W, Shen H-Y. Indoor and outdoor PM2.5 and PM10 concentrations in the air during a dust storm. Build Environ 2010;45:610–4. doi: 10.1016/j.buildenv.2009.07.017. [DOI] [Google Scholar]
- 103. Esmaeil N, Gharagozloo M, Rezaei A et al. . Dust events, pulmonary diseases and immune system. American journal of clinical and experimental immunology 2014;3:20–9. [PMC free article] [PubMed] [Google Scholar]
- 104. Karanasiou A, Moreno N, Moreno T et al. . Health effects from Sahara dust episodes in Europe: literature review and research gaps. Environ Int 2012;47:107–14. doi: 10.1016/j.envint.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 105. Perez L, Tobias A, Querol X et al. . Coarse particles from Saharan dust and daily mortality. Epidemiology 2008;19:800–7. doi: 10.1097/EDE.0b013e31818131cf. [DOI] [PubMed] [Google Scholar]
- 106. Samoli E, Kougea E, Kassomenos P et al. . Does the presence of desert dust modify the effect of PM10 on mortality in Athens, Greece? Sci Total Environ 2011;409:2049–54. doi: 10.1016/j.scitotenv.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 107. Samoli E, Nastos PT, Paliatsos AG et al. . Acute effects of air pollution on pediatric asthma exacerbation: evidence of association and effect modification. Environ Res 2011;111:418–24. doi: 10.1016/j.envres.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 108. Middleton N, Yiallouros P, Kleanthous S et al. . A 10-year time-series analysis of respiratory and cardiovascular morbidity in Nicosia, Cyprus: the effect of short-term changes in air pollution and dust storms. Environ Health 2008;7:39. doi: 10.1186/1476-069x-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zauli Sajani S, Miglio R, Bonasoni P et al. . Saharan dust and daily mortality in Emilia-Romagna (Italy). Occup Environ Med 2011;68:446–51. doi: 10.1136/oem.2010.058156. [DOI] [PubMed] [Google Scholar]
- 110. Zhang X, Lijing Z, Tong DQ et al. . A systematic review of Global Desert dust and associated human health effects. Atmos 2016;7:158. doi: 10.3390/atmos7120158. [DOI] [Google Scholar]
- 111. Lei Y-C, Chan C-C, Wang P-Y et al. . Effects of Asian dust event particles on inflammation markers in peripheral blood and bronchoalveolar lavage in pulmonary hypertensive rats. Environ Res 2004;95:71–6. doi: 10.1016/s0013-9351(03)00136-1. [DOI] [PubMed] [Google Scholar]
- 112. Ichinose T, Nishikawa M, Takano H et al. . Pulmonary toxicity induced by intratracheal instillation of Asian yellow dust (Kosa) in mice. Environ Toxicol Pharmacol 2005;20:48–56. doi: 10.1016/j.etap.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 113. Naota M, Mukaiyama T. Akinori Shimada et al. pathological study of acute pulmonary toxicity induced by intratracheally instilled Asian sand dust (kosa). Toxicol Pathol 2010;38:1099–110. doi: 10.1177/0192623310385143. [DOI] [PubMed] [Google Scholar]
- 114. He M, Ichinose T, Yoshida S et al. . Asian sand dust enhances murine lung inflammation caused by Klebsiella pneumoniae. Toxicol Appl Pharmacol 2012;258:237–47. doi: 10.1016/j.taap.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 115. Ichinose T, Yoshida S, Hiyoshi K et al. . The effects of microbial materials adhered to Asian sand dust on allergic lung inflammation. Arch Environ Contam Toxicol 2008;55:348–57. doi: 10.1007/s00244-007-9128-8. [DOI] [PubMed] [Google Scholar]
- 116. He M, Ichinose T, Yoshida S et al. . Airborne Asian sand dust enhances murine lung eosinophilia. Inhal Toxicol 2010;22:1012–25. doi: 10.3109/08958378.2010.510151. [DOI] [PubMed] [Google Scholar]
- 117. He M, Ichinose T, Song Y et al. . Effects of two Asian sand dusts transported from the dust source regions of Inner Mongolia and Northeast China on murine lung eosinophilia. Toxicol Appl Pharmacol 2013;272:647–55. doi: 10.1016/j.taap.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 118. He M, Ichinose T, Song Y et al. . Desert dust induces TLR signaling to trigger Th2-dominant lung allergic inflammation via a MyD88-dependent signaling pathway. Toxicol Appl Pharmacol 2016;296:61–72. doi: 10.1016/j.taap.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 119. Ichinose T, Sadakane K, Takano H et al. . Enhancement of mite allergen-induced eosinophil infiltration in the murine airway and local cytokine/chemokine expression by Asian sand dust. J Toxicol Environ Health A 2006;69:1571–85. doi: 10.1080/15287390500470833. [DOI] [PubMed] [Google Scholar]
- 120. Hiyoshi K, Ichinose T, Sadakane K et al. . Asian sand dust enhances ovalbumin-induced eosinophil recruitment in the alveoli and airway of mice. Environ Res 2005;99:361–8. doi: 10.1016/j.envres.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 121. Liu B, Ichinose T, He M et al. . Lung inflammation by fungus, Bjerkandera adusta isolated from Asian sand dust (ASD) aerosol and enhancement of ovalbumin-induced lung eosinophilia by ASD and the fungus in mice. Allergy, Asthma, and Clinical Immunology: Official Journal of the Canadian Society of Allergy and Clinical Immunology 2014;10:10. doi: 10.1186/1710-1492-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ren Y, Ichinose T, He M et al. . Aggravation of ovalbumin-induced murine asthma by co-exposure to desert-dust and organic chemicals: an animal model study. Environ Health 2014;13:83. doi: 10.1186/1476-069x-13-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Sadakane K, Ichinose T, Nishikawa M. Effects of co-exposure of lipopolysaccharide and beta-glucan (Zymosan A) in exacerbating murine allergic asthma associated with Asian sand dust. J Appl Toxicol 2019;39:672–84. doi: 10.1002/jat.3759. [DOI] [PubMed] [Google Scholar]
- 124. Naota M, Shiotsu S, Shimada A et al. . Pathological study of chronic pulmonary toxicity induced by intratracheally instilled Asian sand dust (kosa). Toxicol Pathol 2013;41:48–62. doi: 10.1177/0192623312452490. [DOI] [PubMed] [Google Scholar]
- 125. Ichinose T, Yoshida S, Sadakane K et al. . Effects of asian sand dust, Arizona sand dust, amorphous silica and aluminum oxide on allergic inflammation in the murine lung. Inhal Toxicol 2008;20:685–94. doi: 10.1080/08958370801935133. [DOI] [PubMed] [Google Scholar]
- 126. He M, Ichinose T, Kobayashi M et al. . Differences in allergic inflammatory responses between urban PM2.5 and fine particle derived from desert-dust in murine lungs. Toxicol Appl Pharmacol 2016;297:41–55. doi: 10.1016/j.taap.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 127. Chirizzi D, Cesari D, Guascito MR et al. . Influence of Saharan dust outbreaks and carbon content on oxidative potential of water-soluble fractions of PM2.5 and PM10. Atmos Environ 2017;163:1–8. doi: 10.1016/j.atmosenv.2017.05.021. [DOI] [Google Scholar]
- 128. Lovett C, Sowlat MH, Saliba NA et al. . Oxidative potential of ambient particulate matter in Beirut during Saharan and Arabian dust events. Atmospheric environment (Oxford, England: 1994) 2018;188:34–42. doi: 10.1016/j.atmosenv.2018.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Ho KF, Wu K-C, Niu X et al. . Contributions of local pollution emissions to particle bioreactivity in downwind cities in China during Asian dust periods. Environ Pollut 2019;245:675–83. doi: 10.1016/j.envpol.2018.11.035. [DOI] [PubMed] [Google Scholar]
- 130. Kameda T, Azumi E, Fukushima A et al. . Mineral dust aerosols promote the formation of toxic nitropolycyclic aromatic compounds. Sci Rep 2016;6:24427. doi: 10.1038/srep24427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Durant JL, Busby WF, Lafleur AL et al. . Human cell mutagenicity of oxygenated, nitrated and unsubstituted polycyclic aromatic hydrocarbons associated with urban aerosols. Mutat Res-Genet Toxicol 1996;371:123–57. [DOI] [PubMed] [Google Scholar]
- 132. Patton JD, Maher VM, McCormick JJ. Cytotoxic and mutagenic effects of 1-nitropyrene and 1-nitrosopyrene in diploid human-fibroblasts. Carcinogenesis 1986;7:89–93. [DOI] [PubMed] [Google Scholar]
- 133. Esteve W, Budzinski H, Villenave E. Relative rate constants for the heterogeneous reactions of OH, NO2 and NO radicals with polycyclic aromatic hydrocarbons adsorbed on carbonaceous particles. Part 1: PAHs adsorbed on 1-2 μm calibrated graphite particles. Atmos Environ 2004;38:6063–72. [Google Scholar]
- 134. Wang H, Hasegawa K, Kagaya S. The nitration of pyrene adsorbed on silica particles by nitrogen dioxide. Chemosphere 2000;41:1479–84. [DOI] [PubMed] [Google Scholar]
- 135. Kelly FJ, Fussell JC. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmos Environ 2012;60:504–26. doi: 10.1016/j.atmosenv.2012.06.039. [DOI] [Google Scholar]
- 136. Westerling, A., L. Westerling, Anthony Leroy Wildfire Simulations for California’s Fourth Climate Change Assessment: Projecting Changes in Extreme Wildfire Events with a Warming Climate California’s Fourth Climate Change Assessment, California Energy Commission. Merced: University of California, 2018. [Google Scholar]
- 137. Liu JC, Mickley LJ, Sulprizio MP et al. . Particulate air pollution from wildfires in the western US under climate change. Clim Change 2016;138:655–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Ford B, Val Martin M, Zelasky SE et al. . Future fire impacts on smoke concentrations, visibility, and health in the contiguous United States. GeoHealth 2018;2:229–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Watanabe M, Igishi T, Burioka N et al. . Pollen augments the influence of desert dust on symptoms of adult asthma patients. Allergology International: Official Journal of the Japanese Society of Allergology 2011;60:517–24. doi: 10.2332/allergolint.10-OA-0298. [DOI] [PubMed] [Google Scholar]
- 140. Mimura T, Yamagami S, Fujishima H et al. . Sensitization to Asian dust and allergic rhinoconjunctivitis. Environ Res 2014;132:220–5. doi: 10.1016/j.envres.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 141. Baumgartner J, Smith KR, Chockalingam A. Reducing CVD through improvements in household energy: implications for policy-relevant research. Glob Heart 2012;7:243–7. doi: 10.1016/j.gheart.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 142. Ezzati M, Baumgartner JC. Household energy and health: where next for research and practice? Lancet 2017;389:130–2. doi: 10.1016/s0140-6736(16)32506-5. [DOI] [PubMed] [Google Scholar]
- 143. Kalu N, Lufesi N, Havens D et al. . Implementation of World Health Organization integrated Management of Childhood Illnesses (IMCI) guidelines for the assessment of pneumonia in the under 5s in rural Malawi. PLoS One 2016;11:e0155830. doi: 10.1371/journal.pone.0155830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Smith KR, McCracken JP, Weber MW et al. . Effect of reduction in household air pollution on childhood pneumonia in Guatemala (RESPIRE): a randomised controlled trial. Lancet 2011;378:1717–26. doi: 10.1016/s0140-6736(11)60921-5. [DOI] [PubMed] [Google Scholar]
- 145. Mortimer K, Ndamala CB, Naunje AW et al. . A cleaner burning biomass-fuelled cookstove intervention to prevent pneumonia in children under 5 years old in rural Malawi (the cooking and pneumonia study): a cluster randomised controlled trial. Lancet 2017;389:167–75. doi: 10.1016/s0140-6736(16)32507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


