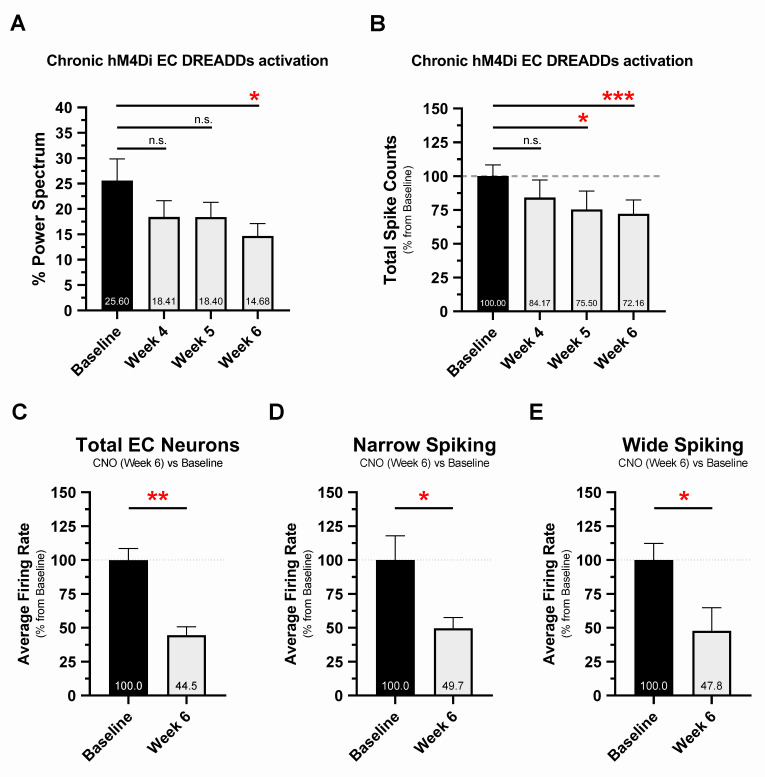
Fig 4. Chronic hM4Di EC DREADDs activation reduces EC neuronal network activity and single-unit firing rates at 6 weeks.
The 16-month EC-Tau/hAPP mice were subjected to 6 weeks of DREADDs activation via osmotic minipump (CNO, 1 mg kg−1 day−1). In vivo neuronal activity was examined at 4, 5, and 6 weeks of CNO treatment and compared with baseline activity (presurgery) measures. A. Chronic hM4Di EC DREADDs activation reduced percentage theta power in EC-Tau/hAPP mice (n = 8 total: n = 4 female, n = 4 male). Repeated-measures ANOVA: F(7,21) = 4.697, p < 0.05. Dunnett’s multiple comparisons test: week 4 versus baseline, p > 0.05; week 5 versus baseline, p > 0.05; week 6 versus baseline, p < 0.05. B. Chronic hM4Di EC DREADDs activation also reduced total spiking activity in EC-Tau/hAPP mice. Repeated-measures ANOVA: F(7,21) = 11.270, p < 0.001. Dunnett’s multiple comparisons test: week 4 versus baseline, p > 0.05; week 5 versus baseline, p < 0.05; week 6 versus baseline, p < 0.001. C. Automated spike sorting and manual cluster cutting was performed on neuronal spiking data from 2 recording sessions per EC-Tau/hAPP mouse (baseline and week 6, CNO). Chronic hM4Di EC DREADDs activation reduced Total EC neuron average firing rates at week 6 versus baseline. Paired t-test: t (6) = 4.972, p = 0.0025. D-E. Single units were then categorized as narrow-spiking or wide-spiking according to spike width. Average firing rates were reduced at week 6 versus baseline in both narrow-spiking EC neurons (Paired t-test: t (6) = 2.635, p = 0.0388) and wide-spiking EC neurons (Paired t-test: t (6) = 2.804, p = 0.0310). Graphical representations appear as mean ± SEM. Normalized data are shown as a percentage of baseline. *p < 0.05, **p < 0.01. ***p < 0.001. Source data are available in S1 Data. Aβ, amyloid-beta; CNO, clozapine-n-oxide; DREADD, designer receptor exclusively activated by a designer drug; EC, entorhinal cortex; hAPP, human amyloid precursor protein; hM4Di, human M4 DREADD receptor; LFP, local field potential; SEM, standard error mean.