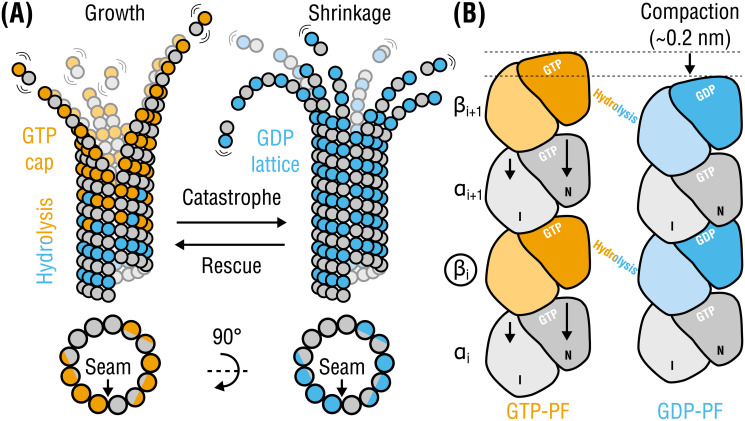
Fig 1. Tubulin life cycle and lattice compaction upon GTP hydrolysis.
(A), Cartoon representation of structural intermediates in MT assembly and disassembly. Individual dimers are composed of α-tubulins (gray circles) and β-tubulins (orange circles when GTP-bound or cyan circles when GDP-bound). Lattice cross-sections (bottom) indicate the location of the seam interface. (B), Local conformational changes proposed to accompany GTP hydrolysis are shown schematically (viewed from within the lumen). Each monomer is illustrated as two domains: intermediate or I and nucleotide-binding or N (C-terminal domains are not shown for simplicity). Rearrangements in α-tubulin around the nucleotide-binding pocket at the inter-dimer interface result in a ∼0.2-nm lattice compaction. The PFs are aligned with respect to monomer βi (marked with a circle). Other more subtle changes (e.g., PF twisting) or intermediate nucleotide states (e.g., GDP-Pi) are not shown for simplicity.