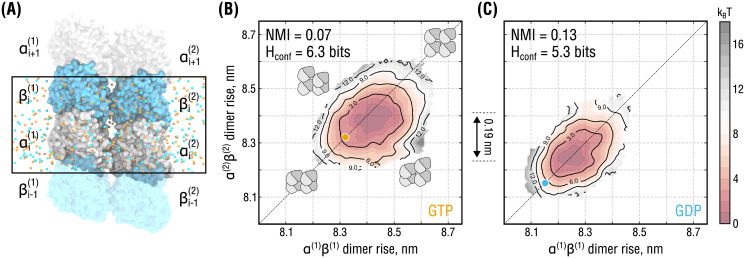
Fig 3. Lateral coupling and nucleotide state affect PF dynamics.
(A), Simulation setup for the double-PF system mimicking a standard (homotypic) lateral interface. Color coding as in Fig 2A. Water molecules are hidden for clarity. Periodic box is marked by a black rectangle. Individual PFs are labeled as (1) and (2). (B) and (C), Free energy surfaces of the system in (A) as a function of dimer rise and nucleotide state obtained by umbrella sampling. The surfaces are color-coded by free energy values with an increment of 1 kBT (dark red to gray). Black solid lines additionally show isoenergetic contours. Orange and cyan circles indicate the dimer rise values observed in the cryo-EM densities of GMPCPP- and GDP-MTs, respectively. Cartooned dimers in (B) schematically show the extreme conformations of the double-PF system in which both are similarly expanded or compacted (along the diagonal) or in conflicting conformations (along the anti-diagonal). The relative shift of 0.19 nm between the minima of the free energy sufraces in (B) and (C) is additionaly indicated.