Abstract
Introduction
Proliferative vitreoretinopathy (PVR), which is regulated by growth factors and cytokines, is the leading cause of failure in vitreoretinal surgery. In this study, we aimed to investigate the role of the human serum and vitreous inflammation-related factors in the development of proliferative vitreoretinopathy (PVR).
Methods
Blood and vitreous samples were obtained from patients undergoing pars plana vitrectomy. Inflammation-related factors were detected using an immunology multiplex assay on a Luminex® xMAP® platform. Patients with PVR and rhegmatogenous retinal detachment (RRD) were compared with macular hole (MH) or epiretinal membrane (ERM) patients without any other ocular or systemic disease.
Results
Thirty-six serum samples and 34 vitreous samples were obtained. Thirty-one different growth factors and cytokines were detected in serum samples. However, none of the circulating growth factors and cytokines were found to be different from the controls. Ten different growth factors and cytokines were measured in the vitreous samples. The concentration levels of PDGF-AA, TGF-α, VEGF, IL-6, IL-8, and TNFβ were found to have significantly increased in the vitreous of PVR patients.
Conclusion
Our study found that none of the circulating inflammation-related factors were changed in PVR or RRD patients, indicating the absence of a system inflammatory biomarkers to predict the development of proliferative vitreoretinopathy. As a supplement to previous research, the concentrations of PDGF-AA, TGF-α, VEGF, IL-6, IL-8, and TNFβ were significantly upregulated in the vitreous of PVR patients. These factors should be considered for preventing PVR.
Keywords: Cytokine, Inflammation, Ophthalmology, Proliferative vitreoretinopathy, Rhegmatogenous retinal detachment, Serum, Vitreous
Key Summary Points
| Proliferative vitreoretinopathy (PVR), which is regulated by growth factors and cytokines, is the leading cause of failure in vitreoretinal surgery. Therefore, new therapeutic targets or early detection and monitoring of biomarkers are needed to prevent PVR. |
| While the role of local retinal inflammation in the development of PVR is documented, we aimed to assess not only the cytokine profile in the vitreous but also the growth factors and cytokine profile in serum to discover new diagnostic markers for evaluating treatment response in PVR patients. |
| Our study for the first time reported the levels of circulating inflammation-related factors in PVR or RRD patients. |
| Our findings show that none of the inflammation-related factors were significantly different in serum, indicating that PVR was only a local retinal inflammation. |
| As a supplement to previous research, the concentrations of PDGF-AA, TGF-α, VEGF, IL-6, IL-8, and TNFβ significantly increased in the vitreous fluid of PVR patients. |
Introduction
Rhegmatogenous retinal detachment (RRD), which is caused by the detachment of the photoreceptor layer from the retinal pigment epithelium, occurs in about 1 in 20,000 people per year [1]. With the improvement of instrumentation and surgical techniques, the cure rate for RRD has improved. However, 5–10% of patients with RD may develop proliferative vitreoretinopathy (PVR), which is the leading cause of vitreoretinal surgery failure and severely impairs visual outcome [2].
Disruption of several inflammation-related factors in the vitreous has been found to be associated with the development and progression of PVR [3]. Inflammatory factors could mediate a wound-healing response involving cell migration, proliferation, epithelial-mesenchymal transformation (EMT), and matrix synthesis, which could result in PVR. Growth factors, such as transforming growth factor β-1 (TGFβ-1), -2, -3, vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF), were found to be significantly upregulated in RRD and PVR patients and proved to be involved in the formation of extracellular matrix (ECM) [4–6]. Other inflammatory cytokines, such as interleukin-6 (IL-6), IL-8, tumor necrosis factor α (TNFα), monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein-1 (MIP-1), and interferon-inducible 10-kDa protein (IP-10), were also upregulated in the vitreous of RRD patients [7–9]. The levels of IL-8 and TGFβ-3 were related to the extent of retinal detachment [5]. The upregulation of growth factors and cytokines, such as TGFβ, VEGF, PDGF, IL-6, IL-8, and TNFα, was proposed as a marker for the formation of PVR [2]. However, no specific factors were found for PVR [10].
Therefore, new therapeutic targets or early detection and monitoring of biomarkers are needed to prevent PVR. Inflammatory factors and retinal imaging could be valuable in monitoring the treatment effect. However, the acquisition of vitreous fluids is an invasive procedure. A circulating biomarker may be more accessible. In this study, we aimed to assess not only the growth factors and cytokine profile in serum but also the cytokine profile in the vitreous to discover possible diagnostic markers for evaluating treatment response in PVR patients.
Methods
Subjects
This is a prospective consecutive case series. This study conformed to the tenets of the Declaration of Helsinki and was approved by the institutional research board of Zhongshan Ophthalmic Center, Sun Yat-sen University. Blood samples for this study were obtained from patients before surgery, and vitreous samples were collected during pars plana vitrectomy in Zhongshan Ophthalmic Center after obtaining informed consent from each patient. Inclusion criteria included all patients undergoing standard three-port pars plana vitrectomy with a single surgeon (Shaochong Zhang) for indications of primary RRD or primary PVR (no prior surgery for RRD) secondary to RRD and epiretinal membranes (EM) or macular hole (MH). Patients with EM or MH were used as control. Patients with systemic diseases (i.e., diabetes, immunologic diseases, infections), uveitis, glaucoma, or any other conditions that might influence the cytokine levels in serum or vitreous were excluded. The extent of PVR was classified according to the Retina Society Terminology Committee, which grades the appearance of PVR into four groups [11]. Primary RRD was defined as early PVR (stage A and B), which combined mild vitreous opacities, retinal stiffening, and curled hole edges.
Collection of Human Samples
For serum preparation, 5 ml peripheral blood was collected into EDTA tubes before surgery, kept still for over 30 min, and then centrifuged at 3000 rpm for 10 min at 4 °C. The supernatant was transferred into a sterile cryogenic vial. All the serum specimens were frozen (− 80 °C) until use.
For vitreous preparation, 200–500 μl undiluted vitreous fluid was collected at the time of pars plana vitrectomy, depending on the different intraocular pressure of each patient. The vitreous fluid was collected with a 2-ml syringe before intraocular infusion using a vitreous cutter. All vitreous specimens were placed on ice right away and transferred into sterile tubes. After centrifugation for 10 min at 4 °C to avoid red blood cells or other confounding cell material, the vitreous sample was transferred to another sterile tube and kept at − 80 °C until assay.
Inflammation-Related Factor Analysis
All the samples were detected using a MILLIPLEX MAP Human Cytokine/Chemokine Magnetic Bead Panel (Millipore-HCYTMAG-60K-PX38) on a Luminex platform according to the manufacturer’s instructions. Twenty-five microliters of serum or vitreous fluid per well was used for analysis. Thirty-one and ten different analytes were examined in serum and vitreous samples, respectively.
Statistical Analysis
SPSS (version 25.0) was used for all statistical analyses. The box plot was used to describe the dispersion of each group. Statistical significance was determined by independent sample t-test between two groups or by one-way analysis of variance (ANOVA) among three groups. To determine whether the data were normally distributed, we used the Shapiro-Wilk test. We used post-hoc Fisher’s least significant difference (LSD) for normally distributed data. If the data were not normally distributed, the post-hoc Tamhane’s T2 was used. P < 0.05 was considered statistically significant. Extreme outliers (> upper quartile + 3 × interquartile range) were excluded from statistical analysis.
Results
Comparisons of Circulating Inflammation-Related Factor Profiles
In total, 36 serum samples were obtained from patients. Patients with MH or EM were chosen as the control group. Patient characteristics are listed in Table 1. To analyze the different growth factors and cytokine profiles between RRD without PVR and severe PVR, we divided patients into three groups (Table 1). Four different growth factors, 12 non-interleukin cytokines, and 15 interleukins were analyzed and compared in the serum sample of the RRD, PVR, and control group (MH or EM) patients (Table 2). None of the 31 factors were found to be significantly different, which indicated that RRD and secondary PVR might be a local inflammation.
Table 1.
Characteristics of the study population
| Serum sample | Control (MH/EM) | RRD | PVR |
|---|---|---|---|
| Number of patients | 13 | 11 | 12 |
| Age (mean ± SD) | 64.38 ± 6.90 | 44.64 ± 14.08 | 56.00 ± 11.68 |
| Gender | |||
| Female | 9, 69% | 3, 27% | 2, 17% |
| Male | 4, 31% | 8, 73% | 10, 83% |
| Duration of the symptoms (days) mean (range) | 488.46 (30–1460) | 17.09 (7–60) | 77.00 (10–210) |
| PVR grade (range) | – | A–B | C–D |
MH macular hole, EM epiretinal membrane, PVR proliferative vitreoretinopathy, RRD rhegmatogenous retinal detachment
Table 2.
Overview of 31 inflammatory factors from serum samples
| Control (pg/ml) mean ± SD | RRD (pg/ml) mean ± SD | PVR (pg/ml) mean ± SD | F | p value (intergroup) | |
|---|---|---|---|---|---|
| EGF | 143.35 ± 93.59 | 156.00 ± 89.80 | 142.58 ± 82.16 | 0.082 | 0.92 |
| FGF-2 | 108.22 ± 105.77 | 75.99 ± 32.59 | 63.28 ± 24.91 | 1.47 | 0.25 |
| TGFα | 6.24 ± 4.87 | 8.94 ± 5.33 | 7.04 ± 4.86 | 0.90 | 0.42 |
| VEGF | 179.31 ± 189.48 | 268.59 ± 403.13 | 84.90 ± 109.75 | 1.30 | 0.29 |
| TNFα | 21.60 ± 9.89 | 19.28 ± 9.19 | 20.08 ± 6.62 | 0.22 | 0.80 |
| Eotaxin | 126.09 ± 53.16 | 109.18 ± 67.44 | 124.08 ± 60.79 | 0.27 | 0.77 |
| GM-CSF | 1.65 ± 0.72 | 1.35 ± 0.60 | 2.37 ± 4.56 | 0.45 | 0.64 |
| IFNα2 | 0.87 ± 1.76 | 3.46 ± 4.91 | 3.35 ± 6.74 | 1.13 | 0.33 |
| IFNγ | 2.60 ± 4.22 | 1.53 ± 0.79 | 5.69 ± 8.15 | 1.86 | 0.17 |
| GRO | 876.11 ± 223.72 | 1094.18 ± 370.09 | 813.84 ± 246.69 | 3.13 | 0.06 |
| MDC | 985.78 ± 221.09 | 1013.55 ± 343.62 | 923.59 ± 465.64 | 0.18 | 0.83 |
| sCD40L | 6289.78 ± 2387.03 | 7584.09 ± 3110.73 | 6756.08 ± 1632.71 | 0.86 | 0.43 |
| IP-10 | 178.55 ± 85.40 | 124.36 ± 58.95 | 155.25 ± 45.22 | 2.00 | 0.15 |
| MCP-1 | 608.95 ± 142.99 | 456.12 ± 156.01 | 517.05 ± 126.18 | 3.12 | 0.06 |
| MIP-1α | 7.47 ± 11.66 | 3.17 ± 4.19 | 6.64 ± 5.81 | 0.91 | 0.41 |
| MIP-1β | 50.03 ± 26.58 | 47.34 ± 31.35 | 53.03 ± 31.22 | 0.11 | 0.90 |
| IL-1α | 0.20 ± 0.21 | 0.13 ± 0.09 | 0.18 ± 0.30 | 0.26 | 0.77 |
| IL-1β | 1.00 ± 0.53 | 0.85 ± 0.34 | 1.32 ± 1.72 | 0.58 | 0.57 |
| IL-2 | 1.18 ± 0.22 | 1.18 ± 0.22 | 1.73 ± 1.83 | 1.06 | 0.36 |
| IL-3 | 0.45 ± 0.19 | 0.43 ± 0.08 | 0.38 ± 0.04 | 0.94 | 0.40 |
| IL-4 | 2.21 ± 5.30 | 3.65 ± 5.18 | 1.19 ± 2.95 | 0.83 | 0.45 |
| IL-5 | 0.76 ± 0.21 | 0.62 ± 0.17 | 0.69 ± 0.32 | 1.07 | 0.36 |
| IL-6 | 0.57 ± 0.88 | 0.27 ± 0.23 | 0.88 ± 2.15 | 0.60 | 0.56 |
| IL-7 | 2.06 ± 1.41 | 2.90 ± 3.49 | 1.61 ± 0.63 | 1.08 | 0.35 |
| IL-8 | 9.81 ± 8.38 | 6.55 ± 5.97 | 8.04 ± 14.47 | 0.30 | 0.74 |
| IL-9 | 0.46 ± 0.21 | 0.45 ± 0.19 | 0.48 ± 0.27 | 0.06 | 0.95 |
| IL-10 | 2.51 ± 5.44 | 1.88 ± 3.96 | 0.53 ± 0.15 | 1.21 | 0.31 |
| IL-12 p70 | 2.03 ± 4.33 | 0.72 ± 1.34 | 0.61 ± 0.43 | 1.05 | 0.36 |
| IL-13 | 0.22 ± 0.29 | 0.18 ± 0.28 | 0.10 ± 0.07 | 0.79 | 0.46 |
| IL-15 | 1.56 ± 0.75 | 1.18 ± 0.31 | 1.34 ± 0.40 | 1.50 | 0.24 |
| IL-17A | 11.95 ± 23.61 | 4.70 ± 7.66 | 3.00 ± 3.41 | 1.26 | 0.30 |
RRD rhegmatogenous retinal detachment, PVR proliferative vitreoretinopathy
Comparisons of Intravitreal Inflammation-Related Factor Profiles
To further study the abnormal intravitreal inflammatory factor profiles in the vitreous of patients with severe PVR, 34 vitreous samples were obtained from patients with MH, EM, and PVR. Patient characteristics are listed in Table 3.
Table 3.
Characteristics of the study population
| Vitreous sample | Control (MH/EM) | PVR |
|---|---|---|
| Number of patients | 10 | 24 |
| Age (mean ± SD) | 63.40 ± 5.83 | 49.12 ± 16.03 |
| Gender | ||
| Female | 6, 50% | 9, 38% |
| Male | 6, 50% | 15, 62% |
| Duration of the symptoms (days) mean (range) | 376.50 (30–1460) | 51.38 (4–240) |
| PVR grade (range) | – | C–D |
MH macular hole, EM epiretinal membrane, PVR proliferative vitreoretinopathy
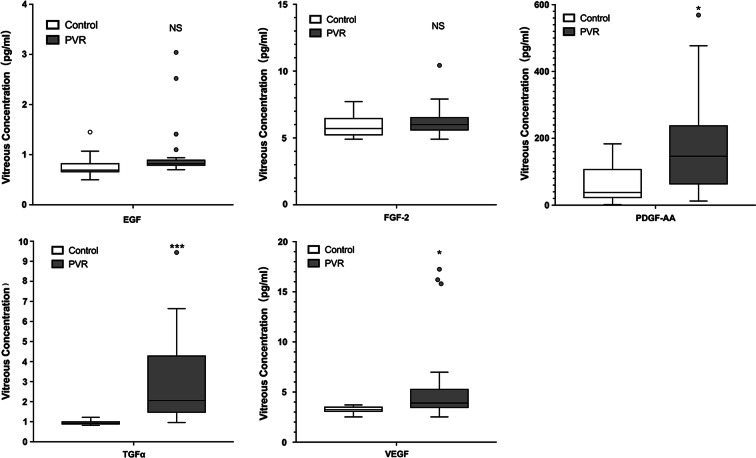
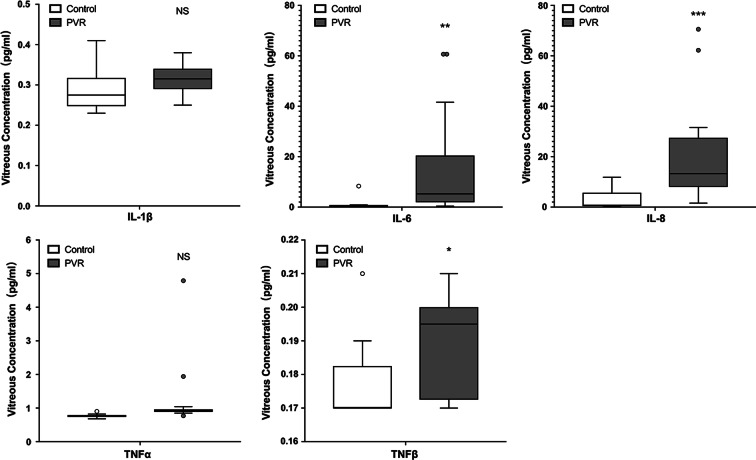
Five different growth factors and five cytokines were measured in the vitreous samples. These factors were involved in the cell proliferation, EMT process, and inflammation. The concentration levels of PDGF-AA, TGF-α, VEGF, IL-6, IL-8, and TNFβ (Table 4) were significantly increased in the vitreous of PVR patients compared with MH or EM patients (Figs. 1, 2).
Table 4.
Overview of ten inflammatory factors from vitreous samples
| Control (pg/ml) mean ± SD | PVR (pg/ml) mean ± SD | p value | |
|---|---|---|---|
| EGF | 0.78 ± 0.28 | 1.01 ± 0.57 | 0.23 |
| FGF-2 | 5.91 ± 0.99 | 6.27 ± 1.14 | 0.39 |
| PDGF-AA | 64.22 ± 59.82 | 179.90 ± 151.92 | 0.027 |
| TGFα | 0.96 ± 0.14 | 3.07 ± 2.19 | 0.00 |
| VEGF | 3.22 ± 0.34 | 5.54 ± 4.32 | 0.015 |
| IL-1β | 0.29 ± 0.053 | 0.32 ± 0.037 | 0.10 |
| IL-6 | 1.25 ± 2.50 | 13.38 ± 18.19 | 0.004 |
| IL-8 | 2.81 ± 3.85 | 18.75 ± 17.37 | 0.00 |
| TNFα | 0.77 ± 0.061 | 1.12 ± 0.81 | 0.19 |
| TNFβ | 0.18 ± 0.013 | 0.19 ± 0.015 | 0.038 |
PVR proliferative vitreoretinopathy
Fig. 1.
Five different growth factors were measured in the vitreous fluid samples. PDGF-AA, TGF-α, and VEGF were significantly increased in the vitreous fluid of proliferative vitreoretinopathy (PVR) patients compared with macular hole (MH) or epiretinal membrane (EM) patients. *p < 0.05, ***p < 0.001, NS not significant
Fig. 2.
Five different cytokines were measured in the vitreous fluid samples. IL-6, IL-8, and TNFβ were significantly increased in the vitreous fluid of proliferative vitreoretinopathy (PVR) patients compared with macular hole (MH) or epiretinal membrane (EM) patients. *p < 0.05, **p < 0.01, ***p < 0.001, NS not significant
Discussion
Previous studies have documented circulating monocytes extravasated rapidly from the vasculature and lining the vitreal surface of the retina following induced RD [12, 13]. Monocyte/macrophage infiltration and activity contributed to inflammation in the progress of PVR. In our study, 31 inflammation-related factors were all found to be insignificantly different in the blood of RRD and PVR patients compared with controls, indicating that no circulating biomarkers could help predict PVR from an early stage of RRD. RRD and secondary PVR might be a local “inflammatory condition.” In our collected vitreous samples, six of the ten measured inflammation-related factors were significantly upregulated in PVR patients. As a supplement to previous research works, the concentrations of TGF-α and TNFβ were found upregulated in our study. The evolution of RRD-induced PVR involved a tissue trauma repair process, which is triggered by the detachment of neuroretina [2]. The photoreceptors started degeneration as soon as the detachment and continued remodeling or apoptosis as long as the photoreceptor layer was detached [14]. In addition, Müller cells, endothelial cells, microglia, astrocytes, and pericytes start to proliferate after retinal detachment [15]. Previous studies indicated that RRD could lead to significant upregulation in a number of growth factors and cytokines in the vitreous. These factors mediated the retinal trauma repair process, which results in PVR. Therefore, finding a circulating biomarker can facilitate the monitoring of the therapeutic effect of the surgery and the prevention of PVR.
Retinal diseases, such as diabetic retinopathy (DR), have been proved to have specific important circulating inflammatory factors. Studies have proved that diabetic macular edema (DME) results from both systemic and local inflammation [16]. TNFα was proposed as an important circulating inflammatory factor in serum in DR [17]. The TNFα level was associated with the risk of non-proliferative DR and DME [18, 19]. IL-6 was also upregulated in both serum and vitreous fluid, which was correlated with DME and progressions of DR [20, 21]. In this study, we aimed to discovered a specific circulating inflammatory factor to predict the severity of the PVR just like the findings in PDR-related studies, which would provide new clinical indicators for the evaluation of the therapeutic effect of PVR. However, we found that the RRD or primary PVR did not affect the levels of inflammatory factors in the blood. This is probably because PDR is a systemic chronic disease and PVR is a local acute inflammation.
Levels of PDGF-AA, TGF-α, VEGF, IL-6, IL-8, and TNFβ were significantly increased in the vitreous of PVR patients in our study. While the other four inflammatory factors (including EGF, FGF-2, IL-1β, and TNFα) were not significantly increased, none were decreased. Among them, PDGF, VEGF, IL-6, and IL-8 have been reported to increase in PVR patients. Like in previous studies, TGF-α and TNFβ were found upregulated in our study. TGF-α is a member of the EGF family, and it binds to EGF receptor (EGFR). TGF-α is reported to be involved in keratinocytes or neural cell proliferation [22, 23], prostate tumorigenesis [24], and angiogenesis [25]. TGF-α enhanced corneal epithelial cell migration by promoting the internalization of EGFR [22]. TGF-α induced EMT in prostate cancer cells [24]. Consistently, this evidence suggests that TGF-α might play a role in PVR formation by promoting both migration and EMT. Secreted by lymphocytes, TNFβ is a member of the TNF superfamily and homologous with TNFα [26]. TNFβ is involved in proinflammatory cascade signaling and apoptotic pathways, which play an important role in inflammatory joint diseases [27, 28]. In our research, TGF-α and TNFβ were significantly upregulated in PVR patients compared with controls, which indicated that TGF-α and TNFβ might participate in the PVR process. Further experiments are needed to clarify the role of TGF-α and TNFβ in PVR.
Conclusions
In summary, our findings show that all the inflammation-related factors were not significantly different in serum, indicating that PVR was only a local retinal inflammation. Adding to previous research, the concentrations of PDGF-AA, TGF-α, VEGF, IL-6, IL-8, and TNFβ significantly increased in the vitreous of PVR patients.
Acknowledgements
Funding
This study was supported by the Fundamental Research Funds of the State Key Laboratory of Ophthalmology (30306020240020312) and Natural Science Foundation of Guangdong Province of China (2019A1515010189). No funding or sponsorship was received for the rapid service fees of this article. The open access fees were funded by the study sponsor.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Yao Ni, Yingyan Qin, Zijing Huang, Fangyuan Liu, Shaochong Zhang, and Zhaotian Zhang declare that they have no conflict of interest.
Compliance with Ethics Guidelines
The study was approved by the Institutional Review Board of Zhongshan Ophthalmic Center (ZOC) affiliated to Sun Yat-sen University (Guangzhou, China) and performed in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.12024765.
Yao Ni and Yingyan Qin contributed equally to this study.
Contributor Information
Shaochong Zhang, Email: zhshaochong@outlook.com.
Zhaotian Zhang, Email: zhangzht9@mail.sysu.edu.cn.
References
- 1.Kwon OW, Song JH, Roh MI. Retinal detachment and proliferative vitreoretinopathy. Dev Ophthalmol. 2016;55:154–162. doi: 10.1159/000438972. [DOI] [PubMed] [Google Scholar]
- 2.Pastor JC, Rojas J, Pastor-Idoate S, Di Lauro S, Gonzalez-Buendia L, Delgado-Tirado S. Proliferative vitreoretinopathy: a new concept of disease pathogenesis and practical consequences. Prog Retin Eye Res. 2016;51:125–155. doi: 10.1016/j.preteyeres.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi S, Adachi K, Suzuki Y, Maeno A, Nakazawa M. Profiles of inflammatory cytokines in the vitreous fluid from patients with rhegmatogenous retinal detachment and their correlations with clinical features. Biomed Res Int. 2016;2016:4256183. doi: 10.1155/2016/4256183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoerster R, Fauser S, Cursiefen C, Kirchhof B, Heindl LM. The influence of systemic renin-angiotensin-inhibition on ocular cytokines related to proliferative vitreoretinopathy. Graefe’s Arch Clin Exp Ophthalmol. 2017;255(9):1721–1725. doi: 10.1007/s00417-017-3707-9. [DOI] [PubMed] [Google Scholar]
- 5.Pollreisz A, Sacu S, Eibenberger K, et al. Extent of detached retina and lens status influence intravitreal protein expression in rhegmatogenous retinal detachment. Invest Ophthalmol Vis Sci. 2015;56(9):5493–5502. doi: 10.1167/iovs.15-17068. [DOI] [PubMed] [Google Scholar]
- 6.Si Y, Wang J, Guan J, Han Q, Hui Y. Platelet-derived growth factor induced alpha-smooth muscle actin expression by human retinal pigment epithelium cell. J Ocul Pharmacol Ther. 2013;29(3):310–318. doi: 10.1089/jop.2012.0137. [DOI] [PubMed] [Google Scholar]
- 7.Nakazawa T, Matsubara A, Noda K, et al. Characterization of cytokine responses to retinal detachment in rats. Mol Vis. 2006;12:867–878. [PubMed] [Google Scholar]
- 8.Nakazawa T, Kayama M, Ryu M, et al. Tumor necrosis factor-alpha mediates photoreceptor death in a rodent model of retinal detachment. Invest Ophthalmol Vis Sci. 2011;52(3):1384–1391. doi: 10.1167/iovs.10-6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garweg JG, Zandi S, Pfister I, Rieben R, Skowronska M, Tappeiner C. Cytokine profiles of phakic and pseudophakic eyes with primary retinal detachment. Acta Ophthalmol. 2019;97(4):e580–e588. doi: 10.1111/aos.13998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pennock S, Haddock LJ, Eliott D, Mukai S, Kazlauskas A. Is neutralizing vitreal growth factors a viable strategy to prevent proliferative vitreoretinopathy? Prog Retin Eye Res. 2014;40:16–34. doi: 10.1016/j.preteyeres.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Hilton G, Machemer R, Michels R, Okun E, Schepens C, Schwartz A. The classification of retinal detachment with proliferative vitreoretinopathy. Ophthalmology. 1983;90(2):121–125. doi: 10.1016/S0161-6420(83)34588-7. [DOI] [PubMed] [Google Scholar]
- 12.Okunuki Y, Mukai R, Pearsall EA, et al. Microglia inhibit photoreceptor cell death and regulate immune cell infiltration in response to retinal detachment. Proc Natl Acad Sci U S A. 2018;115(27):E6264–E6273. doi: 10.1073/pnas.1719601115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Miller EB, Goswami M, et al. Rapid monocyte infiltration following retinal detachment is dependent on non-canonical IL6 signaling through gp130. J Neuroinflammation. 2017;14(1):121. doi: 10.1186/s12974-017-0886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher SK, Lewis GP. Muller cell and neuronal remodeling in retinal detachment and reattachment and their potential consequences for visual recovery: a review and reconsideration of recent data. Vision Res. 2003;43(8):887–897. doi: 10.1016/S0042-6989(02)00680-6. [DOI] [PubMed] [Google Scholar]
- 15.Lewis GP, Charteris DG, Sethi CS, Leitner WP, Linberg KA, Fisher SK. The ability of rapid retinal reattachment to stop or reverse the cellular and molecular events initiated by detachment. Invest Ophthalmol Vis Sci. 2002;43(7):2412–2420. [PubMed] [Google Scholar]
- 16.Figueras-Roca M, Molins B, Sala-Puigdollers A, et al. Peripheral blood metabolic and inflammatory factors as biomarkers to ocular findings in diabetic macular edema. PLoS ONE. 2017;12(3):e0173865. doi: 10.1371/journal.pone.0173865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Preciado-Puga MC, Malacara JM, Fajardo-Araujo ME, et al. Markers of the progression of complications in patients with type 2 diabetes: a one-year longitudinal study. Exp Clin Endocrinol Diabetes. 2014;122(8):484–490. doi: 10.1055/s-0034-1372594. [DOI] [PubMed] [Google Scholar]
- 18.Zorena K, Mysliwska J, Mysliwiec M, et al. Serum TNF-alpha level predicts nonproliferative diabetic retinopathy in children. Mediators Inflamm. 2007;2007:92196. doi: 10.1155/2007/92196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kocabora MS, Telli ME, Fazil K, et al. Serum and aqueous concentrations of inflammatory markers in diabetic macular edema. Ocul Immunol Inflamm. 2016;24(5):549–554. doi: 10.3109/09273948.2015.1034804. [DOI] [PubMed] [Google Scholar]
- 20.Patel JI, Saleh GM, Hykin PG, Gregor ZJ, Cree IA. Concentration of haemodynamic and inflammatory related cytokines in diabetic retinopathy. Eye. 2008;22(2):223–228. doi: 10.1038/sj.eye.6702584. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu E, Funatsu H, Yamashita H, Yamashita T, Hori S. Plasma level of interleukin-6 is an indicator for predicting diabetic macular edema. Jpn J Ophthalmol. 2002;46(1):78–83. doi: 10.1016/S0021-5155(01)00452-X. [DOI] [PubMed] [Google Scholar]
- 22.McClintock JL, Ceresa BP. Transforming growth factor-{alpha} enhances corneal epithelial cell migration by promoting EGFR recycling. Invest Ophthalmol Vis Sci. 2010;51(7):3455–3461. doi: 10.1167/iovs.09-4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai X, Chen J, Xu F, et al. TGFalpha preserves oligodendrocyte lineage cells and improves white matter integrity after cerebral ischemia. J Cereb Blood Flow Metab. 2020;40(3):639–655. doi: 10.1177/0271678X19830791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin W, Pan Y, Zheng X, et al. MicroRNA-124 regulates TGF-alpha-induced epithelial-mesenchymal transition in human prostate cancer cells. Int J Oncol. 2014;45(3):1225–1231. doi: 10.3892/ijo.2014.2506. [DOI] [PubMed] [Google Scholar]
- 25.Klotzsche-von Ameln A, Prade I, Grosser M, et al. PHD4 stimulates tumor angiogenesis in osteosarcoma cells via TGF-alpha. Mol Cancer Res. 2013;11(11):1337–1348. doi: 10.1158/1541-7786.MCR-13-0201. [DOI] [PubMed] [Google Scholar]
- 26.Aggarwal BB, Moffat B, Harkins RN. Human lymphotoxin. Production by a lymphoblastoid cell line, purification, and initial characterization. J Biol Chem. 1984;259(1):686–691. [PubMed] [Google Scholar]
- 27.Calmon-Hamaty F, Combe B, Hahne M, Morel J. Lymphotoxin alpha revisited: General features and implications in rheumatoid arthritis. Arthritis Res Ther. 2011;13(4):232. doi: 10.1186/ar3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buhrmann C, Popper B, Aggarwal BB, Shakibaei M. Resveratrol downregulates inflammatory pathway activated by lymphotoxin α (TNF-β) in articular chondrocytes: comparison with TNF-α. PLoS ONE. 2017;12(11):e0186993. doi: 10.1371/journal.pone.0186993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.