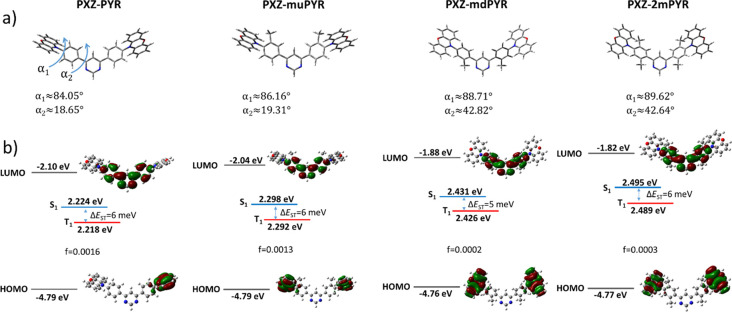
Figure 1.
(a) DFT-optimized molecular geometries of TADF compounds PXZ-PYR, PXZ-muPYR, PXZ-mdPYR, and PXZ-2mPYR. α1 and α2 are dihedral angles between phenyl–phenoxazine and phenyl–pyrimidine units, respectively. (b) HOMO and LUMO energies, S0 → S1/T1 absorption energies with corresponding transition oscillator strengths and S1 – T1 energy gaps (ΔEST) and π-electron density distribution in the HOMO and LUMO of compounds PXZ-PYR, PXZ-muPYR, PXZ-mdPYR, and PXZ-2mPYR.