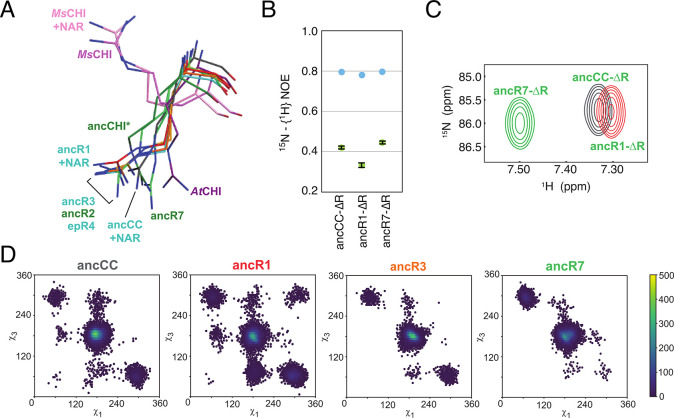
Figure 3.
Changes in the conformational ensemble of the catalytic arginine, R34, during the evolution of chalcone isomerases (CHIs) from binders to catalysts. (A) Structural alignment of crystal structures of different CHIs, showing how widely the conformation of R34 varies. This is in agreement with (B) NMR steady-state heteronuclear NOE values for the catalytic arginine, and the corresponding (C) HSQC signals of the arginine side chain. These indicate both the changes in the mobility of this residue during the evolution of CHI from ancCC to ancR1 to ancR7, as well as the corresponding changes in the electrostatic environment of this side chain. Finally, this is corroborated by long-time-scale molecular dynamics simulations, where the corresponding χ1 and χ3 dihedral angles of R34 during simulation of the different variants once again show the changes in the conformational space of this residue. Reproduced with permission from ref (25). Copyright 2018 Springer Nature.