ABSTRACT
Background
Assessment of amino acid bioavailability is of key importance for the evaluation of protein quality; however, measuring ileal digestibility of dietary proteins in humans is challenging. Therefore, a less-invasive dual stable isotope tracer approach was developed.
Objective
We aimed to test the assumption that the 15N:13C enrichment ratio in the blood increases proportionally to the quantity ingested by applying different quantities of 15N test protein.
Methods
In a crossover design, 10 healthy adults were given a semi-liquid mixed meal containing 25 g (low protein) or 50 g (high protein) of 15N-labeled milk protein concentrate simultaneous with 0.4 g of highly 13C–enriched spirulina. The meal was distributed over multiple small portions, frequently provided every 20 min during a period of 160 min. For several amino acids, the blood 15N- related to 13C-isotopic enrichment ratio was determined at t = 0, 30, 60, 90, 120, 180, 240, 300, and 360 min and differences between the 2 meals were compared using paired analyses.
Results
No differences in 13C AUC for each of the measured amino acids in serum was observed when ingesting a low- or high-protein meal, whereas 15N AUC of amino acids was ∼2 times larger on the high-protein meal (P < 0.001). Doubling the intake of 15N-labeled amino acids increased the 15N:13C ratio by a factor of 2.04 ± 0.445 for lysine and a factor between 1.8 and 2.2 for other analyzed amino acids, with only phenylalanine (2.26), methionine (2.48), and tryptophan (3.02) outside this range.
Conclusions
The amino acid 15N:13C enrichment ratio in the peripheral circulation increased proportionally to the quantity of 15N-labeled milk protein ingested, especially for lysine, in healthy adults. However, when using 15N-labeled protein, correction for, e.g., α-carbon 15N atom transamination is advised for determination of bioavailability of individual amino acids. This trial was registered at www.clinicaltrials.gov as NCT02966704.
Keywords: protein digestibility, stable isotopes, dual-tracer approach, amino acid bioavailability, milk protein, spriulina
Introduction
A critical factor in protein quality assessment is amino acid (AA) bioavailability, which represents the fraction of AA available to the organism after digestion and absorption (1, 2). It is usually derived from ileal AA digestibility—that is, digestibility measured at the terminal ileum after ingestion of a protein. As it is assumed that AAs are absorbed exclusively in the small intestine and that the fraction entering the large intestine is not absorbed in nutritionally relevant quantities (3–5). A promising non-, or minimally, invasive approach for measuring AA bioavailability in humans is the dual stable isotope tracer approach (4, 6, 7).
This dual stable isotope tracer method has been applied for measuring the bioavailability of AAs from different intrinsically labeled proteins in humans (8, 9). The method relies on the ingestion of a meal containing a test protein of unknown AA bioavailability, intrinsically labeled with 2H or 15N, together with a tracer dose of a 13C-labeled protein or standard free AA mixture of known AA bioavailability. The comparison of the differential isotopic enrichment (15N or 2H vs 13C) of each AA in the blood and in the meal reflects, when correcting for label transfer, for example, due to transamination, the relative absorption of each AA that originates from the 2 labeled proteins.
Even though the dual-tracer method has been applied in several studies (6, 8, 10) and its results were comparable with bibliographic true ileal or apparent fecal digestibility values in pigs and humans (9, 11), the approach has not been validated. The approach is based on several assumptions. One of these assumptions is that the 15N:13C (or 2H:13C) enrichment ratio for each AA in blood is identical to the ratio after absorption and that postabsorptive metabolism, including isotopic fractionation, does not influence this ratio. One can test this assumption by varying the quantity of bioavailable protein. Varying the protein quantity provided or its bioavailability should result in a proportional response of this ratio in peripheral blood.
In this study we applied the dual-tracer approach for AA bioavailability using 15N-milk protein concentrate together with 13C-spirulina as test and standard protein, respectively. These protein sources were chosen as their AA digestibility is known (8, 12). The aim was to test the critical assumption of the method that variation in bioavailability should be proportionally reflected in changes in isotope ratios. Therefore, different quantities of test protein were applied to validate whether the 15N:13C enrichment ratio of AAs in the peripheral circulation increased proportionally to the quantity of 15N-labeled milk protein concentrate ingested while keeping the quantity of 13C spirulina identical.
Methods
The experimental protocol was approved by the Medical Ethical Committee of Wageningen University (registered at clinicaltrials.gov: NCT02966704).
Study population
The study population consisted of 10 healthy subjects (5 males, 5 females), with an age between 20 and 35 y. Habitual dietary intake was measured with a food-frequency questionnaire, and general health was assessed using a basic questionnaire. Height and body weight were measured. To be included in the study, subjects needed to have a BMI (kg/m2) between 18.5 and 25 and a habitual dietary protein intake between 10% and 30% of total energy intake. Subjects were excluded when having a history of medical or surgical events or a disease that might influence study outcomes, using medical drugs except for paracetamol, being allergic or intolerant to milk or lactose, when having an alcohol consumption >14 units for females or >21 units for males per week, smoking, using abusive drugs, performing moderate to intense physical activity for >5 h/wk, reporting weight loss or gain of >3 kg in the last month, reporting a slimming or medical diet, or being pregnant or breastfeeding. All subjects gave their written informed consent. The included subjects were 23.5 ± 3.2 y old, had a BMI of 22.2 ± 0.58, and habitual protein intake of 14.4% ± 2.5% of total energy intake.
Study design
A randomized crossover design was applied with 2 different experimental meals, providing either 25 or 50 g of 15N-enriched milk proteins. These meals were provided on 2 different test days, which were separated by 1 wk. Order of the meals was randomized. The day prior to each test day subjects were asked to refrain from vigorous exercise and foods naturally enriched in 13C, like corn or pineapple. Furthermore, subjects were asked to copy their dietary behavior from the first test day to the second test day. For practical reasons, the study was executed in 2 parts: the first part, executed in August 2016, included 4 participants (2 males, 2 females) and the second part, executed in November 2016, included 6 participants (3 males, 3 females).
Experimental meals
To produce 15N-labeled milk protein concentrate, 2 Holstein Friesian dairy cows were rumen-infused with 15N-ammonium sulfate (98% enriched, ∼3.07 g/h per cow; Cambridge Isotope Laboratories, Inc.) and milk protein was extracted. The protein content of the 15N-labeled milk protein concentrate was 67.9% (N × 6.38) and the total 15N enrichment was 2.27 atom percent (AP) or 1.90 atom percent excess (APE), as determined using an elemental analyzer coupled to isotope ration mass spectrometer. The 2 experimental meals consisted of either 25 g or 50 g of 15N-labeled milk protein concentrate providing 18.2 g (low-protein meal) and 35.5 g (high-protein meal) of crude protein, respectively. Both meals contained 400 mg U13C-labeled whole spirulina [>98% enriched (>98AP); Cambridge Isotope Laboratories, Inc.]. The commercially obtained 13C-labeled whole spirulina (>98% enriched; Cambridge Isotope Laboratories, Inc.) consisted of 39.6% (N × 6.25) protein, 2.83% fat, 23.2% neutral detergent fiber, 4.94% ash, and 2.30% moisture. It was produced under quality standards: ISO Guide 34, ISO/IEC 17025, ISO 13485, current good manufacturing practice. The low-protein and high-protein meal also contained 55 g or 32 g dextrine-maltose (Fantomalt; Nutricia), 65 g concentrated lemonade syrup (Karvan cevitam, Cassis), 5.5 g or 5.0 g sunflower oil, 12 g or 7 g locust bean gum (Johannesbroodpitmeel), and 338 g or 341 g water, respectively. Both mixed meals were similar in energy content (513 kcal for the low-protein meal and 514 kcal for high protein) and total weight; moreover, no visual differences in color or viscosity were noticed. Nutrient composition was different between the meals, with the high-protein meal containing 27.8% of energy (E%) protein and 61.9 E% carbohydrates and the low-protein meal contained 14.3 E% protein and 75.4 E% carbohydrates. Both meals contained 10.3 E% fat.
Details of the test day
On the test days, subjects arrived at the human research facilities of Wageningen University after an overnight fast of ≥10 h. After measurement of body weight, a catheter was placed in the antecubital vein and baseline blood samples were taken. Subsequently, the subjects ingested the semi-liquid meal, divided in 9 portions, every 20 min (t = 0, 20, 40, 60, 80, 100, 120, 140, and 160 min). Postprandial blood samples were taken at t = 30, 60, 90, 120, 180, 240, 300, and 360 min after the first portion of the semi-liquid meal was consumed. Blood serum samples were used to determine isotopic enrichment of free AAs. At each time point, 16 mL of blood was collected in two 8-mL SST II Advance blood collection tubes (BD Biosciences). After 30–60 min of clotting, the tubes were centrifuged (1550 × g, 10 min, room temperature) and subsequently serum was placed into aliquots and stored at −80°C until measurement.
15N and 13C AA enrichments
The meal samples were hydrolyzed (6 M HCl at 110°C for 24 h) and filtered (0.22 μm) prior to the extraction. To obtain a proper estimate for meal enrichment, the meals of all study days were analyzed in duplicate. Serum samples were acidified using 1 M HCl and directly mixed with the resin. Serum free AAs were extracted using cation-exchange Dowex AG50×8 resin conditioned under H+-form and eluted with 6 M NH4OH. The purified AAs were analyzed by Gas chromatography combustion isotope ratio mass spectrometry as N(O)-ethoxycarbonyl ethyl ester derivatives. Briefly, 3.2 mL of an ethanol:pyridine solution (80:20, vol:vol) were added to 4 mL of the aqueous sample, the resulting mixture was vortexed before the addition of 400 μL ethyl chloroformate, and the solution gently shaken several times until no bubbles were formed. Then, 2 mL of dichloromethane:hexane (50:50, vol:vol) with 1% ethyl chloroformate (vol:vol) was added and the solution was vigorously vortexed. The upper organic phase was collected and evaporated under stream N2. The obtained residue was diluted in 50 μL of ethylacetate and placed in chromatographic vials, and the samples were stored at −20°C until injection. The 15N and 13C enrichments of derivatized AAs were determined using an Agilent 7890B gas chromatograph (Agilent Technologies) coupled to an Isoprime isotope ratio mass spectrometer (Isoprime; GV Instrument) via the GC5 Isoprime interface. The temperature in the interface was regulated at 350°C and combustion oven was maintained at 930°C and 850°C for 15N and 13C analysis, respectively. A 30-m Rxi-17 capillary column (0.25 mm i.d. and 0.5-μm film thickness; Restek) was used in a constant flow mode, and the high-purity helium was used as the carrier gas at a flow rate of 1.2 mL/min. The inlet temperature was set at 270°C. Samples (2 μL) were injected in splitless mode for 15N analysis and in split mode (10:1) for 13C analysis. The temperature program started at 150°C, increased to 200°C by 4°C/min, and then to 270°C by 25°C/min. The temperature was maintained for 20 min at 270°C. The stable isotopic compositions of nitrogen and carbon were measured using the conventional delta per mill notation: the δ15N and δ13C values were expressed relative to the international standards (AIR-N2 and PDB, respectively). In serum, the enrichment (AP) of 15N and 13C was corrected for the baseline (t = 0) enrichment of each individual AA to obtain APE. To obtain APE, in the meal the enrichment of 15N and 13C was corrected for overall background protein 15N and 13C abundance determined using an elemental analyzer coupled to an isotope ration mass spectrometer and measured in the unlabeled milk collected from cows before the start of label infusion.
Calculations and statistical analyses
The postprandial response in plasma AA 15N and 13C enrichment (APE) is given for each of the analyzed AAs. To quantify the postprandial response, the AUC of plasma AA enrichment over the entire study day (t = 0−360 minutes) was calculated using the trapezoid rule. The AUC was chosen as the main response parameter in the absence of a clear steady state in our experiment.
The blood-to-meal AA 15N- to 13C-enrichment ratio (R1) between the 2 protein sources was calculated according to the following formulas below:
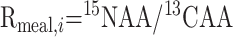 |
(1) |
Rmeal is the meal enrichment ratio of the i AA, with 15N AA and 13C AA the 15N-AA and 13C-AA enrichments of the i AA (in APE).
 |
(2) |
Rblood is the blood enrichment ratio of the i AA, with 15NAUC AA and 13CAUC AA the AUC values of isotopic enrichment of a specific AA (in APE). Finally, R1 is the blood-to-meal 15N- to 13C-enrichment ratio of the i specific AA.
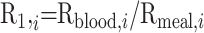 |
(3) |
The R1 value depends for each AA on the ratio between AA digestibility of milk protein and spirulina.
Because of a potential label transfer (e.g., the exchange of 15N, but not of 13C) between indispensable AAs through transamination of the α-carbon 15N, digestibility can either be over- or underestimated using R1. Therefore, the R1 value for each AA was compared with R2 as follows:
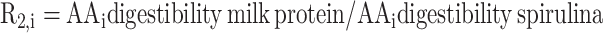 |
(4) |
R2 is the relative digestibility of the i AA between milk protein and spirulina of the i AA as obtained from the literature (8, 12).
Comparing R1 to R2 allows estimation of a transamination correction factor (TcF):
 |
(5) |
When TcF = 1 there is no label transfer due to, for example, transamination; with TcF <1 there is label transfer with a gain in 15N compared with 13C; and with TcF >1 there is label transfer with a reduction in 15N compared with 13C.
All data are expressed as means ± SDs. Differences in enrichment of the 2 meals were tested using a t test with differences being significant when P < 0.05. Within-subject differences in AUC and Rblood values between the 2 test meals for each label and each AA were compared using a paired t test. The 95% CI was used to check if the difference in Rblood between the high- and low-protein meal was 2-fold.
Results
15N- and 13C-AA meal enrichment and Rmeal calculation
15N and 13C enrichment (APE) of AAs in the meal and the ratio 15N:13C enrichment of the AAs in the meal (Rmeal)are given in Table 1. The 15N enrichment was numerically similar in both meals and did not differ significantly for all AAs except for tryptophan and phenylalanine (P < 0.05; Table 1). The 13C enrichment was lower on the high-protein meal compared with the low-protein meal for all AAs, except for lysine (P = 0.057) and methionine (P = 0.128), due to dilution of the 13C-labeled AAs by carbons from the milk protein AAs.
TABLE 1.
Enrichment of individual amino acids in the semi-liquid mixed meals with 25 g (low protein) or 50 g (high protein) of 15N-labeled milk protein concentrate with 0.4 g of 13C-labeled spirulina1
| 15N enrichment (APE) | 13C enrichment (APE) | Rmeal = 15N:13C | ||||||
|---|---|---|---|---|---|---|---|---|
| Low protein | High protein | P | Low protein | High protein | P | Low protein | High protein | |
| Ala | 1.66 ± 0.091 | 1.83 ± 0.058 | >0.05 | 1.33 ± 0.120 | 0.79 ± 0.062 | <0.001 | 1.25 | 2.33 |
| Gly | 1.45 ± 0.084 | 1.58 ± 0.133 | >0.05 | 1.53 ± 0.123 | 0.97 ± 0.061 | <0.001 | 0.95 | 1.63 |
| Val | 1.61 ± 0.050 | 1.65 ± 0.087 | >0.05 | 0.59 ± 0.050 | 0.33 ± 0.022 | <0.001 | 2.70 | 4.96 |
| Leu | 1.53 ± 0.028 | 1.58 ± 0.024 | >0.05 | 0.53 ± 0.049 | 0.30 ± 0.019 | <0.001 | 2.87 | 5.34 |
| Ile | 1.71 ± 0.118 | 1.90 ± 0.060 | >0.05 | 0.62 ± 0.054 | 0.34 ± 0.020 | <0.001 | 2.78 | 5.50 |
| Thr | 1.28 ± 0.063 | 1.60 ± 0.232 | >0.05 | 0.81 ± 0.200 | 0.49 ± 0.133 | <0.05 | 1.58 | 3.28 |
| Phe | 1.53 ± 0.028 | 1.61 ± 0.014 | <0.05 | 0.54 ± 0.047 | 0.30 ± 0.019 | <0.001 | 2.82 | 5.29 |
| Lys | 2.03 ± 0.071 | 2.07 ± 0.022 | >0.05 | 0.49 ± 0.182 | 0.26 ± 0.068 | >0.05 | 4.16 | 7.93 |
| Met | 1.54 ± 0.083 | 1.76 ± 0.141 | >0.05 | 0.46 ± 0.161 | 0.31 ± 0.031 | >0.05 | 3.37 | 5.67 |
| Trp | 0.87 ± 0.027 | 0.94 ± 0.034 | <0.05 | 0.40 ± 0.064 | 0.22 ± 0.026 | <0.01 | 2.20 | 4.20 |
The table shows the mean ± SD of meals provided on different test days, n = 4 for 13C and n = 3 for 15N. APE, atom percent excess.
Appearance of 15N- and 13C-labeled AA in blood and Rblood calculation
The appearance of 13C and 15N enrichment in the blood AA pool (Figure 1) showed that 15N enrichment was numerically lower after ingestion of 25 g compared with 50 g 15N-labeled milk protein concentrate, whereas no visual difference was observed on 13C enrichment. Enrichment of both 13C and 15N AAs steadily increased during consumption of the multiple small portions and peaked at 180 min. In the period after the ingestion of the multiple small portions, towards the end of the study day (t = 240–360 min), a decrease in labeled AAs in blood was observed for most AAs. The AUC 13C and 15N enrichment and Rblood of the different circulating AAs are reported in Table 2. Comparing both meals, no significant differences in the AUC for 13C enrichment for each of the AAs was observed, whereas the AUC of 15N AAs in serum was significantly larger on the high-protein meal for each of the measured AAs, as expected. Consequently, Rblood (15N:13C) was larger after ingestion of the high-protein meal compared with the low-protein treatment for all AAs except for tryptophan. Doubling the intake of 15N-labeled AAs increased the 15N:13C ratio by a factor varying between 1.8 and 2.2 for the analyzed AAs, with only phenylalanine (2.26), methionine (2.48), and tryptophan (3.02) outside this range. For lysine, the fold-difference between the meals was numerically 2. Other AAs tended to deviate numerically from the expected 2-fold difference, even though the factor 2 was in the CI of each AA due to high variation between individuals.
FIGURE 1.
Specific amino acid enrichment in blood of healthy subjects receiving a meal containing 25 g (A and C) or 50 g (B and D) of 15N-labeled milk protein concentrate with 0.4 g of 13C-labeled spirulina. The meal was frequently distributed in 9 discrete portions given every 20 min; the time frame is indicated on top of each graph. Values are means ± SDs, n = 10. APE, atom percent excess.
TABLE 2.
AUCs of amino acid enrichment in blood of 10 healthy subjects receiving 25 g (low protein) or 50 g (high protein) of 15N-labeled milk protein concentrate with 0.4 g of 13C-labeled spirulina1
| 15N AUC | 13C AUC | Rblood = 15N AUC/13C AUC | High:low protein | Lower and upper 95% CI (high:low protein) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low protein | High protein | P | Low protein | High protein | P | Low protein | High protein | P | |||
| Ala | 44.2 ± 7.65 | 83.6 ± 13.1 | <0.001 | 19.6 ± 2.87 | 21.3 ± 4.48 | >0.05 | 2.29 ± 0.471 | 4.00 ± 0.635 | <0.001 | 1.81 ± 0.448 | 1.53, 2.09 |
| Gly | 37.5 ± 8.70 | 69.1 ± 14.7 | <0.001 | 18.1 ± 4.37 | 17.3 ± 5.18 | >0.05 | 2.10 ± 0.411 | 4.33 ± 0.656 | <0.001 | 2.10 ± 0.580 | 1.70, 2.50 |
| Val | 82.9 ± 14.1 | 151.6 ± 25.0 | <0.001 | 37.9 ± 7.65 | 33.3 ± 5.38 | >0.05 | 2.21 ± 0.273 | 4.57 ± 0.434 | <0.001 | 2.11 ± 0.440 | 1.84, 2.38 |
| Leu | 62.9 ± 11.5 | 125.0 ± 18.9 | <0.001 | 32.4 ± 4.51 | 29.8 ± 4.83 | >0.05 | 1.94 ± 0.271 | 4.22 ± 0.319 | <0.001 | 2.21 ± 0.398 | 1.97, 2.46 |
| Ile | 73.4 ± 15.9 | 142.3 ± 25.1 | <0.001 | 48.3 ± 7.91 | 42.7 ± 5.94 | >0.05 | 1.51 ± 0.194 | 3.33 ± 0.381 | <0.001 | 2.19 ± 0.358 | 1.95, 2.42 |
| Thr | 42.9 ± 10.7 | 89.6 ± 20.5 | <0.001 | 27.1 ± 5.53 | 26.6 ± 5.16 | >0.05 | 1.75 ± 0.453 | 3.46 ± 0.953 | <0.001 | 1.92 ± 0.588 | 1.45, 2.39 |
| Phe | 63.6 ± 12.4 | 125.7 ± 27.8 | <0.001 | 28.6 ± 4.84 | 25.8 ± 5.27 | >0.05 | 2.23 ± 0.368 | 4.91 ± 0.785 | <0.001 | 2.26 ± 0.599 | 1.89, 2.64 |
| Lys | 108 ± 15.9 | 190 ± 17.1 | <0.001 | 19.9 ± 2.65 | 17.8 ± 4.05 | >0.05 | 5.33 ± 0.300 | 11.0 ± 2.02 | <0.001 | 2.04 ± 0.445 | 1.75, 2.33 |
| Met | 72.6 ± 20.2 | 153 ± 36.2 | <0.001 | 9.3 ± 7.80 | 14.6 ± 16.0 | >0.05 | 10.0 ± 4.69 | 19.5 ± 12.3 | <0.05 | 2.48 ± 1.858 | 1.10, 3.85 |
| Trp | 31.6 ± 7.39 | 62.1 ± 3.55 | <0.001 | 7.91 ± 7.50 | 7.68 ± 4.50 | >0.05 | 5.26 ± 3.35 | 10.0 ± 4.51 | >0.05 | 3.02 ± 3.021 | 0.376, 5.67 |
Values are means ± SDs unless otherwise indicated, n = 10. The meal was frequently distributed in 9 discrete portions given every 20 min. Rblood for both meals and the fold-difference between these 2 meals are shown.
Blood-to-meal 15N:13C enrichment ratio (R1) and estimation of 15N transamination for each AA (TcF)
R1 for AAs was in the range from 0.544 to 3.44 (Table 3). For the different AAs, R1 depends on the difference in the relative absorption of the 15N- and 13C-labeled AAs from milk and spirulina protein, respectively, but also depends on label transfer (e.g., on the level of transamination of the 15N atom on the α-carbon). For each circulating AA, the ratio R1 was compared with the “reference” R2, which is the milk protein to spirulina ratio from AA digestibility values reported in the literature (8, 12). The R2 values ranged from 1.00 to 1.23 (Table 3). When AAs do not undergo label transfer such as transamination, as known for lysine and threonine, R1 is expected to be not different from the ratio R2. When there is a loss or a gain in 15N:13C atom enrichment due to metabolism such as α-carbon transamination or homocysteine remethylation, deviations of R1 above or below the value of R2 are expected. This was expressed by calculating transamination correction factors (TcFs = R1/R2), which ranged from 0.42 to 2.11 for the different AAs (Table 3). TcFs reported in this study are an estimation and represent the potential correction required for the R1 values to calculate AA digestibility of the 15N-labeled test protein using the 15N:13C dual-tracer approach.
TABLE 3.
Comparison of current data with the literature for estimation of TcF1
| Digestibility, % | R1 = Rblood/Rmeal | TcF = R2/R1 | |||||
|---|---|---|---|---|---|---|---|
| Milk2 | Spirulina3 | R2 | Low protein | High protein | Low protein | High protein | |
| Ala | 95.9 | — | — | 1.84 ± 0.377 | 1.72 ± 0.273 | — | — |
| Gly | 99.3 | — | — | 2.21 ± 0.434 | 2.66 ± 0.403 | — | — |
| Val | 93.4 | 87.1 | 1.07 | 0.82 ± 0.101 | 0.92 ± 0.087 | 1.32 ± 0.155 | 1.17 ± 0.100 |
| Leu | 95.0 | 86.0 | 1.10 | 0.68 ± 0.094 | 0.79 ± 0.060 | 1.65 ± 0.235 | 1.40 ± 0.100 |
| Ile | 95.0 | 84.2 | 1.13 | 0.54 ± 0.070 | 0.61 ± 0.069 | 2.11 ± 0.321 | 1.89 ± 0.204 |
| Thr | 95.4 | 82.5 | 1.16 | 1.11 ± 0.287 | 1.05 ± 0.291 | 1.10 ± 0.273 | 1.18 ± 0.313 |
| Phe | 94.9 | 95.3 | 1.00 | 0.79 ± 0.131 | 0.93 ± 0.148 | 1.29 ± 0.224 | 1.10 ± 0.159 |
| Lys | 95.6 | 77.5 | 1.23 | 1.28 ± 0.072 | 1.39 ± 0.254 | 0.96 ± 0.053 | 0.91 ± 0.162 |
| Met | 91.6 | 84.1 | 1.09 | 2.97 ± 1.390 | 3.44 ± 2.170 | 0.42 ± 0.145 | 0.57 ± 0.544 |
| Trp | — | — | — | 2.39 ± 1.521 | 2.37 ± 1.074 | — | — |
Values are means ± SDs unless otherwise indicated, n = 10. The blood-to-meal 15N/13C ratios (R1) obtained in healthy participants who received 25 g (low protein) or 50 g (high protein) of 15N-labeled milk protein concentrate with 0.4 g of 13C-labeled spirulina are shown. Literature data on milk protein and spirulina amino acid digestibility were used to calculated R2 and subsequently TcF. TcF, transamination correction factor.
Data from reference 12
Data from reference 8.
Discussion
The dual-tracer approach has been proposed to measure food protein AA bioavailability by using a labeled (15N or 2H) test protein together with a tracer dose of labeled (13C) standard protein or AA mixture in a test meal (4). This approach was shown to be a minimal invasive technique to measure dietary AA digestibility in adults, children, and cystic fibrosis patients (6, 10, 13). This dual-tracer approach was applied using 2H-labeled test protein in combination with 13C-labeled standard spirulina (8, 9, 11) as well as with 15N-labeled spirulina protein in combination with 2H-labeled phenylalanine (6). In this context, the present study evaluated the potential of using 15N-labeling of milk protein with 13C spirulina for measuring dietary protein AA bioavailability in humans. Variation in bioavailability should be proportionally reflected in changes in isotope ratios. The validity of this approach is difficult to test with protein sources differing in bioavailability as, by definition, variations in bioavailability between such sources and methodology (dual isotope technique vs. true ileal AA disappearance) have to be combined. To circumvent this problem, we tested this crucial assumption by doubling the provision of a highly digestible protein source.
We evaluated the effect of the quantity of 15N-labeled test milk protein when applying the dual-tracer method on blood isotopic 15N and 13C-AA enrichment by providing 25 g or 50 g of 15N-labeled milk protein concentrate with 0.4 g of 13C-labeled spirulina. Doubling the quantity of milk protein provided was intended to double the quantity absorbed. Increasing the quantity of protein in a meal does not have detrimental effects on the proportion digested and absorbed, as previous studies showed that increasing protein content up to 400 g/kg dry matter intake did not affect true ileal crude protein digestibility in rats (14) and pigs (15), nor did crude protein content affect true ileal AA digestibility in pigs (16). Moreover, milk protein is an easily digestible protein, with true ileal digestibility values >90% (12). Thus, increasing the quantity provided will also result in proportional AA absorption and thereby a good strategy to test whether the measured blood isotopic 15N:13C ratio is not affected by postabsorptive metabolism.
No difference was observed in the 13C enrichment of circulating AAs between the low- and high-protein meal, which both contained an equal quantity of 13C-labeled spirulina. As expected, the 15N AUC was higher after ingestion of the high-protein meal compared with the low-protein meal. Most importantly, the 2-fold difference for Rblood between these meals for lysine confirmed our idea that the 15N:13C ratio in the blood is proportional to the quantity of AAs absorbed. Furthermore, for all other AAs no significant deviation from 2 was observed, although this might be related to the high variation, as phenylalanine, methionine, and tryptophan are numerically very deviating. Thereby the results validate a crucial assumption in the application of the dual-isotope approach that the isotopic appearance of AAs is proportional to the absorbed quantities, especially for lysine. Thus, potential differences in postabsorptive metabolism for 15N- and 13C-labeled AAs are not affected by the quantity absorbed (7). This observation is also critical for the future application of the dual-tracer approach as it was unclear whether the relative digestibility estimated with the dual-tracer approach would be affected by a quantity of protein provided.
In previous dual-tracer studies, similar protein quantities (21–24 g vs 18.2 g in the present study for the low-protein meal) were provided in a meal with higher energy content (714–834 kcal vs this study's meal of 513 kcal) (9, 11). Therefore, our study's protein-to-energy ratio was only slightly higher for the low-protein meal (14.3 E% protein) but markedly higher for the present high-protein meal (27.8 E% protein) compared with other dual-tracer studies. As it is well known that the amount of carbohydrates in a protein meal can influence the retention of AAs in the body (17), this could theoretically have influenced postabsorptive metabolism of labeled AAs and, in the case of isotopic fractionation, result in deviating labeled AA fluxes (18). However, here we showed that postabsorptive metabolism of labeled AAs does not influence the proportional appearance of labeled lysine and potentially other AAs.
Unlike earlier work using the dual-tracer approach (8) we used the combination of 15N test protein and 13C reference protein to test the critical assumption of proportional isotope ratios. Depending on the protein source, labeling with 15N is more convenient and efficient than 2H labeling, although it comes with additional challenges. In the dual-isotope approach with a 13C-labeled reference protein, there is for most AAs no exchange of 13C for the 13C-AAs absorbed from the reference 13C-labeled protein, and the 13C enrichment of circulating AAs is only related to its digestibility and absorption, except for methionine. In contrast, for the 15N-labeled test protein, the 15N enrichment of circulating AAs is proportional to the digestibility and absorption of the AA from the test protein but also to the level of α-carbon 15N atom transamination of the AAs. Transamination is a complex process of AA metabolism that is active in different tissues and differently affects AAs (19–21). Assuming that transamination is the main source of discrepancy between predicted (i.e., R2) and observed relative digestibility (i.e., R1) between our 2 protein sources, TcFs could be estimated. The TcF was calculated by comparing the relative digestibility measured in this study (R1) with that calculated from data in the literature (R2), although these literature values were determined with different methods and in different populations (8, 12). The values of the transamination factors ranged from 0.42 to 2.11 for 15N atom transamination. It is important to realize that these TcFs are much higher than those calculated for loss of deuterium (2H) from indispensable AAs that ranged from 1.002 to 1.081 (8). This is unavoidable because only 1 hydrogen atom out of multiple hydrogen atoms on the AA molecule is lost during transamination in comparison with the loss (or gain) of the only nitrogen atom in many AAs. The estimated TcFs >1 indicate that for the branched-chain AAs valine, leucine, and isoleucine 15N compared with 13C is lost. This is substantiated by the fact that, even though the transamination of branched-chain AAs is reversible, the likeliness of re-amination of the α-keto-acid with labeled nitrogen is virtually nil because of the rate of the process and the large amino-nitrogen pool size (18, 22). In contrast, for methionine, a transamination factor <1 was estimated from our data, suggesting an increased 15N relative to 13C, which may be caused by homocysteine remethylation (23). From our results it is clear that measuring the AA bioavailability of a test protein using 15N-labeling of the test protein requires a specific correction factor for most AAs. The rate of label transfer due to metabolism such as transamination and the appropriate correction factors for future studies need to be quantified before the dual-tracer approach can be routinely applied using 15N-labeled proteins. However, for AAs that do not undergo transamination, lysine and threonine, a correction factor for transamination may be less critical for the right determination of AA bioavailability than for other indispensable AAs such as the branched-chain AAs.
Our results also indicate other aspects that are essential for future applications of the dual-tracer approach. The Rmeal was expected to be 2 times larger for the high-protein meal compared with the low-protein meal. The enrichments measured in our meal showed a high level of variation, especially for 13C, most likely due to flaws in the homogeneity of the meal and subsampling portions for analysis, as mixing small quantities of highly labeled spirulina within a semi-liquid meal does not necessarily lead to a homogeneous distribution. Moreover, the values differed slightly from our expectations; however, this could also be due to uncertainty in AA enrichment of spirulina and the unknown AA content and enrichment of the thickening agent. As the primary aim of the current study was not to calculate digestibility of the test protein, this uncertainty in our meal data did not influence our observation that the 15N:13C enrichment ratio in the blood increased proportionally to the quantity of 15N-labeled protein ingested. However, it affected the size of the blood-to-meal ratio (R1) and the TcF values; therefore, no quantitative comparisons between low- and high-protein meals in R1 of TcF were made.
The pattern of frequently feeding small meals prevented the potential effect of large differences in the intestinal kinetics (24, 25) between spirulina and milk protein and led to a parallel kinetic response of isotope enrichment in blood AAs. Accordingly, the pattern of 13C and 15N enrichment of circulating AAs over time showed quite similar profiles, although with a slightly faster decrease in 15N enrichment after the last meal. However, we did not obtain an isotopic plateau, probably because of a too-short meal feeding period or a lack of an initial priming bolus as applied by Devi et al. (8). Due to a lack of plateau, AUC was chosen for calculations. However, it is uncertain whether this issue, analytical variations for the chosen isotopic labels, or day-to-day subject variation resulted in the relatively high variation that was observed in Table 2. Moreover, the high variation for some AAs may have caused a lack in statistical power to show differences due to the quantity of test protein. As a result, the estimated TcFs also show high variability, especially compared with the previously reported <1% variation in 2H TcFs (8).
The calculations with the dual-tracer approach rely on multiple ratios; therefore, small deviations in isotopic enrichment in blood samples may have large effects on the final result of the digestibility calculation. For example, a 5% increase or decrease in 15N or 13C enrichment in the blood alone could result in an ∼10% increase or ∼10% decrease in R1, and with similar variations in the meal enrichment this will be even larger. A slightly higher SD in mean indispensable AA digestibility value was also shown by Kashyap et al. (9), where digestibility values obtained with the dual-tracer approach were compared with bibliographic true ileal or apparent fecal digestibility values in pigs and humans. This potential variation should be taken into account when designing studies.
In conclusion, this study demonstrated that the 15N:13C enrichment ratio of AAs in the peripheral circulation increased proportionally to the quantity of 15N-labeled milk protein concentrate ingested, especially for lysine, indicating that postabsorptive metabolism is similar for an AA with either a 13C or 15N label, and that the ratio of labels in the blood is proportional and thereby representative for absorption. This illustrates the validity of the dual-isotope method to measure relevant variation in the bioavailability.
However, applying this dual-tracer approach with 15N to determine bioavailability of AAs from food proteins requires correction factors that are especially critical for AAs with high transamination rates and less essential for AAs that do not undergo transamination like threonine and lysine. The correction factors estimated in the current study need further quantification and validation before application.
Acknowledgments
The authors’ responsibilities were as follows—DT, MM, CG, and WJJG: designed the research; NvdW, NVK, and JC: conducted the research; NvdW and MM: analyzed data; NvdW, MM, and DT: wrote the manuscript; and all authors: read and approved the final manuscript.
Notes
This work was supported by the Dutch Dairy Association.
Author disclosures: DT is a member of the Journal's Editorial Board. The authors report no conflicts of interest.
The funders had no role in data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations used: AA, amino acid; AP, atom %; APE, atom % excess; E%, % of energy; TcF, transamination correction factor.
Contributor Information
Nikkie van der Wielen, Division of Human Nutrition, Wageningen University and Research, Wageningen, Netherlands; Animal Nutrition Group, Wageningen University and Research, Wageningen, Netherlands.
Nadezda V Khodorova, UMR Physiologie de la nutrition et du comportement alimentaire, AgroParisTech, National Research Institute for Agriculture, Food and Environment, Université Paris-Saclay, Paris, France.
Walter J J Gerrits, Animal Nutrition Group, Wageningen University and Research, Wageningen, Netherlands.
Claire Gaudichon, UMR Physiologie de la nutrition et du comportement alimentaire, AgroParisTech, National Research Institute for Agriculture, Food and Environment, Université Paris-Saclay, Paris, France.
Juliane Calvez, UMR Physiologie de la nutrition et du comportement alimentaire, AgroParisTech, National Research Institute for Agriculture, Food and Environment, Université Paris-Saclay, Paris, France.
Daniel Tomé, UMR Physiologie de la nutrition et du comportement alimentaire, AgroParisTech, National Research Institute for Agriculture, Food and Environment, Université Paris-Saclay, Paris, France.
Marco Mensink, Division of Human Nutrition, Wageningen University and Research, Wageningen, Netherlands.
References
- 1. Tome D, Jahoor F, Kurpad A, Michaelsen KF, Pencharz P, Slater C, Weisell R. Current issues in determining dietary protein quality and metabolic utilization. Eur J Clin Nutr. 2014;68:537–8. [DOI] [PubMed] [Google Scholar]
- 2. Tome D. Digestibility issues of vegetable versus animal proteins: protein and amino acid requirements—functional aspects. Food Nutr Bull. 2013;34:272–4. [DOI] [PubMed] [Google Scholar]
- 3. Fuller MF, Tomé D. In vivo determination of amino acid bioavailability in humans and model animals. J AOAC Int. 2005;88:923–34. [PubMed] [Google Scholar]
- 4. Lee WT, Weisell R, Albert J, Tome D, Kurpad AV, Uauy R. Research approaches and methods for evaluating the protein quality of human foods proposed by an FAO Expert Working Group in 2014. J Nutr. 2016;146:929–32. [DOI] [PubMed] [Google Scholar]
- 5. van der Wielen N, Moughan PJ, Mensink M. Amino acid absorption in the large intestine of humans and porcine models. J Nutr. 2017;147(8):1493–8. [DOI] [PubMed] [Google Scholar]
- 6. Engelen MP, Com G, Anderson PJ, Deutz NE. New stable isotope method to measure protein digestibility and response to pancreatic enzyme intake in cystic fibrosis. Clin Nutr. 2014;33:1024–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tome D. Editorial on “Measurement of protein digestibility in humans by a dual tracer method”—a key limiting factor of protein quality. Am J Clin Nutr. 2018;107:855–6. [DOI] [PubMed] [Google Scholar]
- 8. Devi S, Varkey A, Sheshshayee MS, Preston T, Kurpad AV. Measurement of protein digestibility in humans by a dual-tracer method. Am J Clin Nutr. 2018;107:984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kashyap S, Shivakumar N, Varkey A, Duraisamy R, Thomas T, Preston T, Devi S, Kurpad AV. Ileal digestibility of intrinsically labeled hen's egg and meat protein determined with the dual stable isotope tracer method in Indian adults. Am J Clin Nutr. 2018;108:980–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kashyap S, Shivakumar N, Varkey A, Preston T, Devi S, Kurpad AV. Co-ingestion of black tea reduces the indispensable amino acid digestibility of hens' egg in Indian adults. J Nutr. 2019;149:1363–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kashyap S, Varkey A, Shivakumar N, Devi S, Reddy BHR, Thomas T, Preston T, Sreeman S, Kurpad AV. True ileal digestibility of legumes determined by dual-isotope tracer method in Indian adults. Am J Clin Nutr. 2019;110(4):873–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gaudichon C, Bos C, Morens C, Petzke KJ, Mariotti F, Everwand J, Benamouzig R, Dare S, Tome D, Metges CC. Ileal losses of nitrogen and amino acids in humans and their importance to the assessment of amino acid requirements. Gastroenterology. 2002;123:50–9. [DOI] [PubMed] [Google Scholar]
- 13. Shivakumar N, Kashyap S, Kishore S, Thomas T, Varkey A, Devi S, Preston T, Jahoor F, Sheshshayee MS, Kurpad AV. Protein-quality evaluation of complementary foods in Indian children. Am J Clin Nutr. 2019;109:1319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Donkoh A, Moughan PJ. The effect of dietary crude protein content on apparent and true ileal nitrogen and amino acid digestibilities. Br J Nutr. 1994;72:59–68. [DOI] [PubMed] [Google Scholar]
- 15. Bax ML, Buffiere C, Hafnaoui N, Gaudichon C, Savary-Auzeloux I, Dardevet D, Sante-Lhoutellier V, Remond D. Effects of meat cooking, and of ingested amount, on protein digestion speed and entry of residual proteins into the colon: a study in minipigs. PLoS One. 2013;8:e61252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fan MZ, Sauer WC, McBurney MI. Estimation by regression analysis of endogenous amino acid levels in digesta collected from the distal ileum of pigs. J Anim Sci. 1995;73:2319–28. [DOI] [PubMed] [Google Scholar]
- 17. Deutz NE, Ten Have GA, Soeters PB, Moughan PJ. Increased intestinal amino-acid retention from the addition of carbohydrates to a meal. Clin Nutr. 1995;14:354–64. [DOI] [PubMed] [Google Scholar]
- 18. Waterlow JC. Protein turnover. Wallingford (England): CABI Publishing; 2006. [Google Scholar]
- 19. Braun A, Vikari A, Windisch W, Auerswald K. Transamination governs nitrogen isotope heterogeneity of amino acids in rats. J Agric Food Chem. 2014;62:8008–13. [DOI] [PubMed] [Google Scholar]
- 20. Macko SA, Estep MLF, Engel MH, Hare P. Kinetic fractionation of stable nitrogen isotopes during amino acid transamination. Geochim Cosmochim Acta. 1986;50:2143–6. [Google Scholar]
- 21. Jungas RL, Halperin ML, Brosnan JT. Quantitative-analysis of amino-acid oxidation and related gluconeogenesis in humans. Physiol Rev. 1992;72:419–48. [DOI] [PubMed] [Google Scholar]
- 22. Matthews DE, Bier DM, Rennie MJ, Edwards RH, Halliday D, Millward DJ, Clugston GA. Regulation of leucine metabolism in man: a stable isotope study. Science. 1981;214:1129–31. [DOI] [PubMed] [Google Scholar]
- 23. Blom HJ, Smulders Y. Overview of homocysteine and folate metabolism: with special references to cardiovascular disease and neural tube defects. J Inherit Metab Dis. 2011;34:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrere B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci USA. 1997;94:14930–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lacroix M, Leonil J, Bos C, Henry G, Airinei G, Fauquant J, Tome D, Gaudichon C. Heat markers and quality indexes of industrially heat-treated [15N] milk protein measured in rats. J Agric Food Chem. 2006;54:1508–17. [DOI] [PubMed] [Google Scholar]



