Abstract
The long-term decline of monarch butterflies has been attributed to loss of their milkweed (Asclepias sp.) host-plants after the introduction of herbicide-tolerant crops. However, recent studies report pesticide residues on milkweed leaves that could act as a contributing factor when ingested as part of their larval diet. In this study, we exposed monarch larvae to six pesticides (insecticide: clothianidin; herbicides: atrazine, S-metolachlor; fungicides: azoxystrobin, pyraclostrobin, trifloxystrobin) on their primary host-plant, A. syriaca. Each was tested at mean and maximum levels reported from published analyses of milkweeds bordering cropland and thus represent field-relevant concentrations. Monarch lethal and sub-lethal responses were tracked over their complete development, from early instar larvae to adult death. Overall, we found no impact of any pesticide on immature development time and relatively weak effects on larval herbivory or survival to adulthood. Comparatively stronger effects were detected for adult performance; namely, a 12.5% reduction in wing length in response to the fungicides azoxystrobin and trifloxystrobin. These data collectively suggest that monarch responses to host-plant pesticides are largely sublethal and more pronounced in the adult stage, despite exposure only as larvae. This outcome has important implications for risk assessment and the migratory success of monarchs in North America.
Subject terms: Zoology, Ecology, Environmental sciences, Natural hazards
Introduction
Agricultural intensification has reduced the abundance and diversity of pollinators such as bees and butterflies in many regions around the world1–3. While this reduction is partly due to the indirect effect of habitat loss (i.e., large fields with frequent herbicide inputs have fewer floral resources), intensification can also have direct effects by exposing individuals to pesticide residues that increase mortality or impair development. This is particularly the case for insects developing on flowering plants growing along crop field edges, where pesticide drift or runoff is unavoidable. Several studies now confirm the presence of non-target pesticides in diets of pollinators foraging on non-crop, wild plants4–9. Much of these efforts focus on neonicotinoid insecticides due to their rapid adoption by farmers since the 1990s10, resulting in a dramatically higher toxic load for pollinators in agricultural landscapes11,12.
As a correlative pattern, the abundance of many butterflies has declined with the rise in neonicotinoid use over time13–15, but experimental studies testing the impacts of pesticides on non-target butterflies are, in general, limited16. For example, a systematic review of four butterfly families—Lycaenidae, Nymphalidae, Hesperiidae, Papilionidae—revealed few published data evaluating the influence of non-target insecticides17. In addition, the limited cases overwhelmingly center on insecticides, with comparatively few incorporating fungicides or herbicides. This research gap is noteworthy since some experiments show negative effects of herbicides on Lepidoptera performance, including reduced survival or wing size18–20, adult emergence21 and sex-pheromone production22. Similar studies, mainly on commercial (Bombyx mori L.) and pest (Spodoptera litura F.) lepidopteran species, have found that fungicides can also reduce larval survival, pupal weight or adult longevity23,24. Moreover, fungicides synergize the action of insecticides by inhibiting cytochrome P450 enzymes in honey bees25–28. Comparable work in fruit flies revealed that otherwise nontoxic herbicides greatly increase the lethality of insecticides when applied together29. Thus, the lack of data on non-insecticidal chemistries, either alone or in combination, prevents a broader assessment of pesticide toxicity for non-target Lepidoptera inhabiting agroecosystems.
A secondary challenge in disentangling the role of pesticides in butterfly population ecology is that chemicals can act on either the larval or adult stage. Although it is assumed that adults may encounter spray drift during flight or residues when contacting pesticide-contaminated surfaces or foods (nectar; e.g.,30), less attention is paid to larval host-plants, which could form the bulk of exposure over their full lifecycle31–33. Little is known about pesticide concentrations on non-crop plant leaves and how these translate to changes in caterpillar development. Notably, dietary exposure as a larva can have delayed, sublethal effects on adult butterflies. The survival of cabbage butterfly, Pieris brassicae, larvae was unaffected by wide variation (up to 200 ppb) of the neonicotinoid imidacloprid in their diet, but even the lowest dose (1 ppb) reduced adult wing size34.
The monarch butterfly, Danaus plexippus (Lepidoptera: Nymphalidae), is an iconic species whose population is declining in North America. Numerous causal factors have been implicated in their decline35–37, with disappearance of its larval host-plant, milkweed (Asclepias sp.), due to the introduction of the herbicide glyphosate being the leading hypothesis38–43. Some have noted, however, that the decline temporally coincides with the introduction and growing popularity of neonicotinoids and thus speculate that insecticides or other agrochemicals could act as a contributing factor. To date, only a few studies have experimentally assessed the impact of agricultural pesticides on monarch larvae, providing some pieces of the puzzle. Yet, these studies suffer from methodological limitations that prevent us from extrapolating to the broader question of monarch decline. All used ornamental milkweeds that are planted in home gardens such as tropical milkweed A. curassavica44,45 or swamp milkweed A. incarnata46,47, rather than the common milkweed A. syriaca, which is the most abundant and widely used larval host-plant for eastern migratory monarchs48,49. This discrepancy is potentially important since milkweed species differ dramatically in defensive traits affecting host-plant suitability50 (e.g., latex, trichomes); for instance, tropical and swamp milkweeds are notable for having extremely high and low levels of cardenolides, respectively51. Similarly, experiments mostly employ brief ‘pulsed’ exposure periods with larvae consuming a known insecticide dose over a 24–48 h period45,46. This approach is useful in defining acute toxicity and calculating LD50 values but does not accurately portray natural exposure where larvae consume trace amounts over their entire developmental period. Additionally, all prior studies investigated insecticides; none have tested other pesticide types, e.g., fungicides, herbicides.
Here, we evaluated the effects of six pesticides—the insecticide clothianidin; the herbicides atrazine and S-metolachlor; the fungicides azoxystrobin, pyraclostrobin and trifloxystrobin—and their combination on monarch development. These were among the most commonly detected chemicals on A. syriaca leaves in a 2-year field survey of milkweeds bordering cropland52. Because we know their specific concentrations, we tested each at mean and maximum levels; thus, all data represent field-realistic pesticide types and doses. Importantly, we exposed monarchs to these treatments on A. syriaca leaves over their complete larval period, measuring both immediate (i.e., larval mortality) and delayed responses (i.e., changes in adult size and longevity). These data are ultimately used in risk assessment to estimate the likelihood of lethal or nonlethal effects and their potential to harm monarchs in the wild.
Methods
Plants and insects
All experiments were performed using the common milkweed, A. syriaca. Seeds (obtained from Prairie Moon Nursery, Winona, MN) were surface sterilized by soaking in a 5% bleach solution, rinsed with distilled water, and the seed coat was nicked with a razor. Seeds were then germinated on moist filter paper in a parafilm-sealed petri dish, covered with aluminum foil and stored at 4 °C for 1–2 weeks, after which they were moved to a growth chamber at 28 °C for 3–4 days. Seedlings were placed in propagation trays filled with SunGro horticulture germination mix with Osmocote 14-14-14 (ICL Specialty Fertilizers), before being moved to a greenhouse and transplanted into 366 mL pots filled with SunGro professional growing mix with two teaspoons of time-released NPK fertilizer (Scotts Osmocote Classic). Biocontrol agents (Koppert Biological Systems)—lacewing (Chrysoperla carnea) larvae, predaceous mites (Amblyseius cucumeris), entomopathogenic nematodes (Steinernema feltiae)—were released on plants regularly to control a diversity of milkweed pests (e.g., thrips, aphids, fungus gnats) without using pesticides.
Monarch eggs were the offspring of wild-caught butterflies from the eastern migratory population. Gravid females were either obtained locally in Indiana or from individuals collected in Georgia at Emory University, provided by Jacobus de Roode.
Pesticide treatments
We used a larval exposure bioassay using six of the most commonly detected pesticides from our recent field survey52. For each, we tested the mean and maximum concentrations recorded to remain within biologically plausible limits. The specific treatments and target concentrations (ng/g) were: clothianidin (mean = 15.28, max = 56.55), atrazine (mean = 8.59, max = 238.7), S-metolachlor (mean = 1.23, max = 15.31), azoxystrobin (mean = 0.67, max = 31.06), pyraclostrobin (mean = 8.51, max = 211.75), and trifloxystrobin (mean = 4.48, max = 164.25). In addition, we included a mixture treatment that included all six pesticides together at mean/max concentrations. These pesticide treatments were compared against two controls—an untreated and a solvent (acetone) application to control for the fact that pesticides were diluted in a carrier—resulting in 16 total treatments [= (6 individual pesticides × 2 concentrations) + (1 mixture × 2 concentrations) + 2 controls].
We simulated dietary exposure by applying pesticide treatments to milkweed leaves that were subsequently fed to monarch larvae (see below section); however, individuals were also likely exposed topically as they crawled on the leaf surface and, thus, we cannot truly differentiate oral from contact. This exposure route was used, rather than applying compounds directly to the exterior of the insect cuticle, because some of the pesticides are systemic and likely taken up by plant roots (e.g., clothianidin), in which case monarchs come into contact with active ingredients while feeding on contaminated leaves.
To experimentally treat monarchs at the target dose, we harvested leaves from potted, greenhouse-grown milkweeds. After cutting at the base of the petiole, we rinsed leaves in distilled water, and removed leaf cores (20 mm diameter) using a cork borer, avoiding major leaf veins. Leaf discs were temporarily stored at 4 °C before being treated and collected daily to avoid wilting or biochemical degradation.
High purity pesticides (ordered from Sigma-Aldrich or ChemService, Inc.)—clothianidin (99.0%), atrazine (98.1%), S-metolachlor (97.6%), azoxystrobin (99.5%), pyraclostrobin (99.9%), trifloxystrobin (99.4%)—were diluted in 1 mL of acetone as an initial stock, from which we created subsequent serial dilutions in acetone to a final volume of 15 mL. Acetone was used because the pesticides readily dissolve in it and it evaporates quickly from the leaf surface. Target pesticide concentrations (ng/g) were adapted to the average sized milkweed leaf disc (65.7 mg) and applied to the abaxial surface in 20 μL aliquots using an Eppendorf repeater pipette. Treated discs were left to dry in the fume hood for 30 min to ensure that the acetone had fully evaporated, before being used to feed monarch larvae. Discs were handled with forceps specific to each pesticide treatment to avoid cross-contamination.
The above-described methodology was also used in a related study on monarch choice, during which we used LC/MS analysis of treated leaf discs to confirm that the target concentrations were achieved. This analysis (data reported in53) demonstrated that all six of the pesticides tested were at levels within ca. 5% of the field value that applications were based on. Clothianidin mean/max treatments, for instance, were recorded at 14.36 and 54.47 ng/g, respectively, from treated leaf discs; targeted concentrations from field data were 15.28 and 56.55 ng/g.
Monarch development
This experiment was conducted in two separate trials; the first, was from June 30 to July 17, 2017 and the second from September 11 to October 2, 2017. Each trial contained 5 biological replicates of each treatment, with a replicate being considered an individual caterpillar, reared from the neonate to adult stage (n = 180 total monarchs across trials). Experimental larvae were reared in a greenhouse on the Purdue University campus (West Lafayette, Indiana, USA); although this environment was warmer and more variable (i.e., temperature fluctuations) than laboratory growth chambers, we noticed that monarchs perform better when reared under natural light conditions.
Recently hatched larvae were randomly assigned to a pesticide treatment and weighed using a semi-micro balance, before being placed in an individually labeled 5.5 oz plastic container with a damp strip of paper for humidity and 2–3 cm incision in the lid to provide some ventilation. Containers were arranged in a randomized complete block design, with one full replicate of each concentration (i.e., 9 treatments) grouped together. The spatial arrangement of blocks within the greenhouse was also randomized and rotated several times throughout the experiment to reduce minor environmental differences due to positional effects. Containers were kept within a PVC frame covered with shade cloth to reduce direct sunlight and a fan operating at low speed to cool the area even further. Each day, we added a set of newly treated leaf discs, recorded larval survival, removed frass, replaced paper strips, and cleaned containers with water. The number of discs added per container daily increased over time as larvae grew and required more leaf tissue. Any leaf disc fragments that had not been eaten were taken out and photographed under a transparent 4 × 4 mm grid to later quantify herbivory per day per caterpillar.
Upon completion of the larval stage, pupae were weighed on an analytical balance and moved to mesh cages (95.25 × 57.15 × 59.69 cm LWD) separated by treatment. Cardboard platforms were placed at the top of cages to provide shade and a structure to attach pupae. Pupal survival and development time (i.e., no. days until adult emergence) were recorded.
When adults emerged, their date of emergence and sex were recorded. After their wings dried, but before they started flying, a small amount of paint from Painters’ opaque paint markers (Elmer’s Products, Inc.) was applied to the hindwing with a fine paintbrush to track butterflies by date of emergence and register the day of death. A different paint color was used each day to differentiate between emergence dates. Individuals that emerged the same day were marked with the same color. Butterflies were fed in cages with banana and tangerine slices, water, and Gatorade54,55, which were replaced every other day. To minimize temperature-induced stress (> 30 °C), cages were misted with cold water and fans were placed on nearby benches to increase airflow. Cages were checked daily and adult longevity was calculated as time from butterfly emergence from their pupae until death.
After a butterfly died, date of death was recorded and its size was measured by folding the wings together and measuring from the farthest tip of the forewing to the first white dot on the thorax56. There is a linear relationship between forewing length and size in monarchs57 and butterflies in general58.
Statistical analysis
Larval survival across treatments was analyzed using Kaplan–Meier survival curves and the log-rank test to compare among treatments. A posthoc pairwise comparison between the control and each treatment was done only for maximum concentrations. Based on sample sizes, a 50% reduction in survival (5/10 mortality) would be necessary to detect a significant (P < 0.05) effect of individual pesticide treatments. These analyses were performed using the package survivalAnalysis59 in R.
A two-way ANOVA was used to test the effects of trial and pesticide treatments on the response variables: leaf area consumed (herbivory), immature development time (larval + pupal development), pupal weight, adult longevity, and adult size (wing length). Univariate ANOVAs were performed for each response variable with separate analyses conducted for the mean and maximum concentrations. Normality assumptions were verified using QQ plots and homogeneity of variances using Levene’s test. A post hoc pairwise t-test comparison was used to test differences in adult size between the control and other treatments. The Holm–Bonferroni p_adjusted method was used to control the overall Type I error. Analyses were performed in R 3.6.2 using the packages tidyverse60, ggpubr61, rstatix62, car63 and ggplot264.
We also used the package ggcorrplot65 to analyze and graph the correlation matrix between monarch performance variables: larval development time, herbivory, pupal weight, adult size, and adult longevity. This analysis combined individuals from all treatment groups and was performed separately on monarchs reaching adulthood, reared at mean and maximum pesticide concentrations for trials 1 and 2.
Although raw data are provided in Table 1, figures were created using effect sizes66 to control for random trial variation by standardizing treatment data against the control in each block, using the formula: ln(treatment/control). We used the R package pairwiseCI67 to calculate 95% confidence intervals surrounding control vs. individual pesticide treatment comparisons to estimate uncertainty in our findings (Supplementary Table 1).
Table 1.
Raw data showing the sample size (n), mean, and variation (SE) for monarch responses at mean (A) and maximum (B) pesticide concentrations tested.
| Treatments | n | Herbivory (cm2) | Pesticide consumed (ng) | n | Immature developm. (# days) | n | Pupal weight (g) | n | Adult longevity (# days) | Adult wing size (cm) |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean + SE | Mean + SE | Mean + SE | Mean + SE | Mean + SE | Mean + SE | |||||
| (A) Mean concentrations | ||||||||||
| Control | 10 | 210 + 15.2 | 0 + 0 | 9 | 21.9 + 1.47 | 9 | 1.41 + 0.14 | 8 | 10.1 + 1.26 | 4.65 + 0.08 |
| Acetone | 9 | 247 + 26.8 | 0 + 0 | 9 | 23.8 + 1.89 | 9 | 1.18 + 0.14 | 9 | 10.6 + 1.76 | 4.27 + 0.17 |
| Clothianidin | 9 | 221 + 18.5 | 66.4 + 5.5 | 8 | 23.6 + 1.76 | 8 | 1.14 + 0.10 | 8 | 10.5 + 1.76 | 4.59 + 0.12 |
| Atrazine | 8 | 224 + 24 | 45.5 + 4.9 | 8 | 22.2 + 1.56 | 8 | 1.45 + 0.15 | 8 | 17.1 + 4.13 | 4.44 + 0.10 |
| S-Metolachlor | 10 | 248 + 24.6 | 7.05 + 0.7 | 10 | 22.8 + 1.47 | 10 | 1.2 + 0.12 | 10 | 12.5 + 1.86 | 4.44 + 0.13 |
| Azoxystrobin | 8 | 227 + 24.2 | 2.28 + 0.2 | 7 | 23.6 + 1.99 | 7 | 1.08 + 0.13 | 6 | 15.0 + 2.71 | 4.07 + 0.07 |
| Pyraclostrobin | 8 | 234 + 26.2 | 57.4 + 6.4 | 8 | 22.6 + 1.66 | 8 | 1.08 + 0.13 | 7 | 16.4 + 1.76 | 4.26 + 0.07 |
| Trifloxystrobin | 7 | 207 + 27.9 | 26.8 + 3.6 | 6 | 19.7 + 1.67 | 6 | 1.55 + 0.19 | 5 | 21.6 + 4.98 | 4.08 + 0.12 |
| Mix | 7 | 243 + 25.6 | 156 + 16.4 | 7 | 24.7 + 1.51 | 7 | 1.08 + 0.18 | 5 | 13.4 + 2.73 | 4.44 + 0.16 |
| (B) Max. concentrations | ||||||||||
| Control | 10 | 251 + 23 | 0 + 0 | 10 | 22.8 + 1.49 | 10 | 1.41 + 0.13 | 10 | 14.6 + 4.20 | 4.64 + 0.05 |
| Acetone | 7 | 206 + 20.3 | 0 + 0 | 6 | 22 + 1.81 | 6 | 1.55 + 0.17 | 5 | 16.2 + 4.47 | 4.34 + 0.30 |
| Clothianidin | 7 | 283 + 28.3 | 322 + 32.3 | 7 | 24.7 + 1.76 | 7 | 1.2 + 0.17 | 7 | 10.2 + 1.96 | 4.29 + 0.11 |
| Atrazine | 8 | 217 + 23.8 | 1,495 + 164 | 7 | 22.4 + 1.99 | 7 | 1.32 + 0.16 | 6 | 15.0 + 3.29 | 4.1 + 0.24 |
| S-Metolachlor | 9 | 230 + 21.1 | 75.1 + 6.88 | 9 | 22.6 + 1.67 | 9 | 1.45 + 0.18 | 9 | 14.6 + 2.27 | 4.44 + 0.10 |
| Azoxystrobin | 5 | 209 + 32.1 | 101 + 15.6 | 5 | 20 + 1.52 | 5 | 1.44 + 0.21 | 5 | 14.9 + 3.11 | 4.46 + 0.10 |
| Pyraclostrobin | 10 | 247 + 25.2 | 1,225 + 125 | 8 | 22 + 1.7 | 8 | 1.5 + 0.163 | 8 | 15.0 + 1.56 | 4.4 + 0.10 |
| Trifloxystrobin | 6 | 250 + 28.4 | 827 + 93.7 | 6 | 23.3 + 1.96 | 6 | 1.29 + 0.18 | 6 | 13.3 + 2.43 | 4.52 + 0.13 |
| Mix | 9 | 217 + 20.7 | 3,450 + 329 | 9 | 22.2 + 1.67 | 9 | 1.42 + 0.17 | 9 | 11.5 + 0.89 | 4.36 + 0.08 |
Data combine trials 1 and 2.
Results
We started the experiment with 180 individuals (= 9 treatments × 2 concentrations × 10 biological replicates), from which 33 died as larvae, 8 died during the pupal stage, and 8 either died during adult eclosion or emerged with deformed wings and thus were removed from the analysis. This developmental mortality resulted in 73% of monarchs surviving to a fully functioning adult across all treatments.
Of those who survived to adulthood, we found significant trait correlations across life stages for several pairwise comparisons (Supplementary Fig. 1). While the presence and strength of these relationships varied among trials, the most consistent—3 out of 4 tests—were positive associations between larval development time and herbivory (i.e., larvae who lived longer, ate more; r = 0.44–0.51) and pupal weight and wing length (i.e., heavier cocoons produced adults with bigger wings; r = 0.40–0.71).
Larval survival
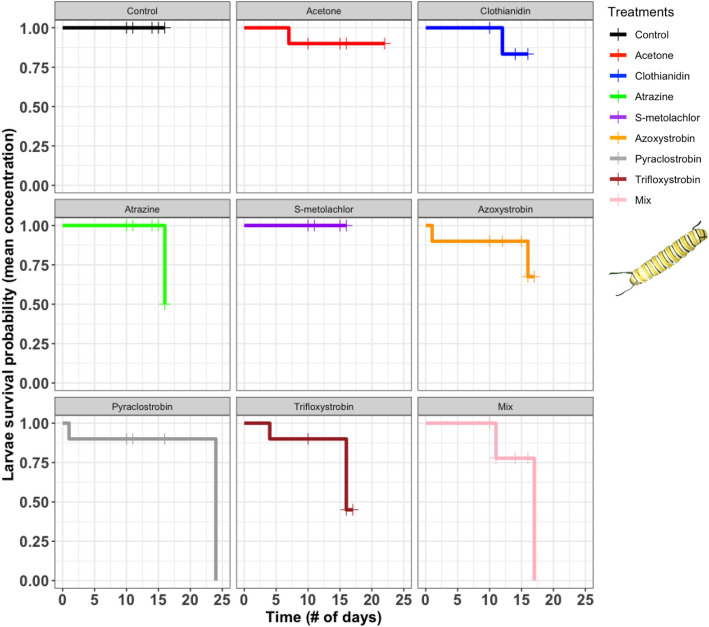
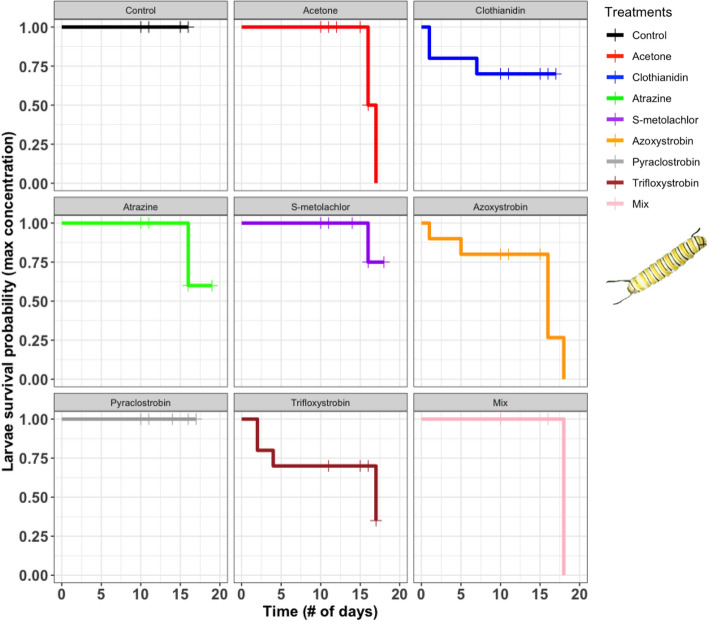
None of the pesticide treatments affected the daily survival of monarch larvae when ingested at their mean concentrations (log-rank = 0.65; Fig. 1). However, pesticides at their maximum concentrations had a marginally significant impact on larval survival (log-rank = 0.064; Fig. 2). Pairwise comparisons between survival curves revealed a significant difference only between the control and azoxystrobin (50% survival; χ2 = 4.6, P = 0.03) with marginal negative effects caused by trifloxystrobin (60% survival; χ2 = 3.4, P = 0.07) and clothianidin (70% survival; χ2 = 3.4, P = 0.07). In the untreated control, 100% of larvae (20 out of 20) survived to pupation across both trials.
Figure 1.
Survival curves comparing daily larval survival of monarchs in response to the untreated control, solvent control (acetone), six pesticides, and their combination (mix) at mean concentrations. Cross hatches on curves represent larvae that pupated. Photo credit: Paola Olaya-Arenas.
Figure 2.
Survival curves comparing daily larval survival of monarchs in response to the untreated control, solvent control (acetone), six pesticides, and their combination (mix) at maximum concentrations. Cross hatches on curves represent larvae that pupated. Photo credit: Paola Olaya-Arenas.
Herbivory
Similar to larval survival, there was no effect of pesticides on the amount of leaf tissue consumed by monarch larvae when presented at their mean concentrations (Table 2A); however, at maximum concentrations there was a marginal (P = 0.06) impact on herbivory (Table 2B). Specifically, atrazine or the mixture of all pesticides combined caused a 14% reduction in leaf consumption (Supplementary Fig. 2). Trial strongly affected herbivory—but did not interact with treatment—primarily because we increased the number of leaf discs from 10 to 20 between trials 1 and 2 to ensure that food was not limiting for larvae.
Table 2.
Results from ANOVA testing the main and interactive effects of trial and pesticide treatment on monarch development at mean (A) and maximum (B) concentrations.
| Response variables | Predictor variables | df | F | P | ges |
|---|---|---|---|---|---|
| (A) Mean concentrations | |||||
| Herbivory | Trial | 1.58 | 262.17 | 0.00 | 0.82 |
| Pesticide | 8.58 | 1.60 | 0.15 | 0.18 | |
| Trial × pesticide | 8.58 | 1.38 | 0.23 | 0.16 | |
| Immature development | Trial | 1.54 | 1692.76 | 0.00 | 0.97 |
| Pesticide | 8.54 | 1.15 | 0.35 | 0.15 | |
| Trial × pesticide | 8.54 | 1.68 | 0.12 | 0.20 | |
| Pupal weight | Trial | 1.54 | 113.17 | 0.00 | 0.68 |
| Pesticide | 8.54 | 1.59 | 0.15 | 0.19 | |
| Trial × pesticide | 8.54 | 1.46 | 0.19 | 0.18 | |
| Adult longevity | Trial | 1.49 | 0.00 | 0.96 | 0.00 |
| Pesticide | 8.49 | 2.02 | 0.06 | 0.25 | |
| Trial × pesticide | 7.49 | 1.93 | 0.09 | 0.22 | |
| Wing length | Trial | 1.49 | 18.67 | 0.00 | 0.28 |
| Pesticide | 8.49 | 2.78 | 0.01 | 0.31 | |
| Trial × pesticide | 7.49 | 0.37 | 0.92 | 0.05 | |
| (B) Max. concentrations | |||||
| Herbivory | Trial | 1.53 | 595.81 | 0.00 | 0.92 |
| Pesticide | 8.53 | 2.02 | 0.06 | 0.23 | |
| Trial × pesticide | 8.53 | 1.02 | 0.43 | 0.13 | |
| Immature development | Trial | 1.49 | 1658.36 | 0.00 | 0.97 |
| Pesticide | 8. 49 | 0.69 | 0.70 | 0.10 | |
| Trial × pesticide | 8. 49 | 0.78 | 0.62 | 0.11 | |
| Pupal weight | Trial | 1.49 | 149.60 | 0.00 | 0.75 |
| Pesticide | 8. 49 | 0.61 | 0.76 | 0.09 | |
| Trial × pesticide | 8. 49 | 0.53 | 0.83 | 0.08 | |
| Adult longevity | Trial | 1.47 | 0.83 | 0.37 | 0.02 |
| Pesticide | 8.47 | 0.32 | 0.96 | 0.05 | |
| Trial × pesticide | 8.47 | 0.51 | 0.84 | 0.08 | |
| Wing length | Trial | 1.47 | 15.47 | 0.00 | 0.25 |
| Pesticide | 8.47 | 1.85 | 0.09 | 0.24 | |
| Trial × Pesticide | 8.47 | 0.93 | 0.50 | 0.14 | |
The generalized eta squared (ges) calculates how much variation in the response is explained by predictor variables. Significant (P < 0.05) or marginally significant (P < 0.1) effects are bolded for emphasis.
Development time
Most monarchs developed through their immature (larval + pupal) stage in 19–25 days (Table 1). None of the pesticide treatments at either concentration affected this developmental rate (Table 2).
Pupal weight
As with development time, the weight of monarch pupae was unaffected by any of the pesticide treatments at both concentrations tested (Tables 1, 2).
Adult longevity
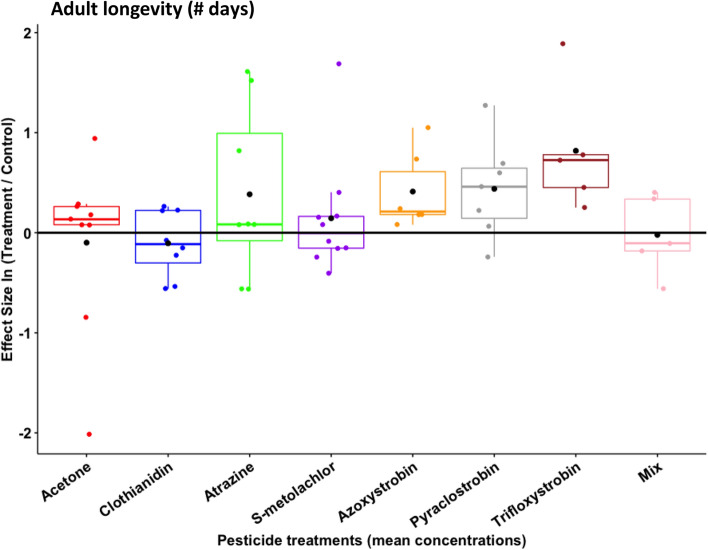
Butterflies lived in captivity for approximately 10–22 days (Table 1). Pesticide treatment had a marginally significant (P = 0.06) effect on adult longevity at mean concentrations (Table 2A), but there was no impact at maximum concentrations (Table 2B). Interestingly, adult longevity was extended after larvae were reared on certain pesticides at the lower concentration used; most notably, monarchs reared with exposure to the three fungicides—azoxystrobin, pyraclostrobin, trifloxystrobin—lived for 50–100% longer than control individuals (Fig. 3).
Figure 3.
Butterfly adult longevity (# of days living), after being reared on milkweed leaves treated with the solvent control (acetone) and those experimentally treated to simulate the mean concentrations of field values for six pesticides and their combination (mix). An effect size at zero—i.e., the black horizontal line—represents no quantitative difference between the treatment and control, whereas positive or negative values indicate relative increases or decreases, respectively. Box plots show median values with 95% confidence intervals. Black circles are the treatment mean and colored circles are individual data points.
Adult size
Monarch wing length ranged from 4 to 5 cm (Table 1) and was the most consistently affected response variable with a significant pesticide treatment effect at mean concentrations (Table 2A) and marginally significant effect (P = 0.09) at maximum concentrations (Table 2B). In virtually all cases, wing size tended to be lower when reared as larvae with pesticide residues; however, after a Holm–Bonferroni correction, individual effects of azoxystrobin and trifloxystrobin were significant, with a 12.5% reduction in wing length compared to the control (Fig. 4A). While these fungicides also trended toward lower wing length at maximum concentrations, atrazine-reared monarchs developed to the smallest final size of all treatments (Fig. 4B).
Figure 4.
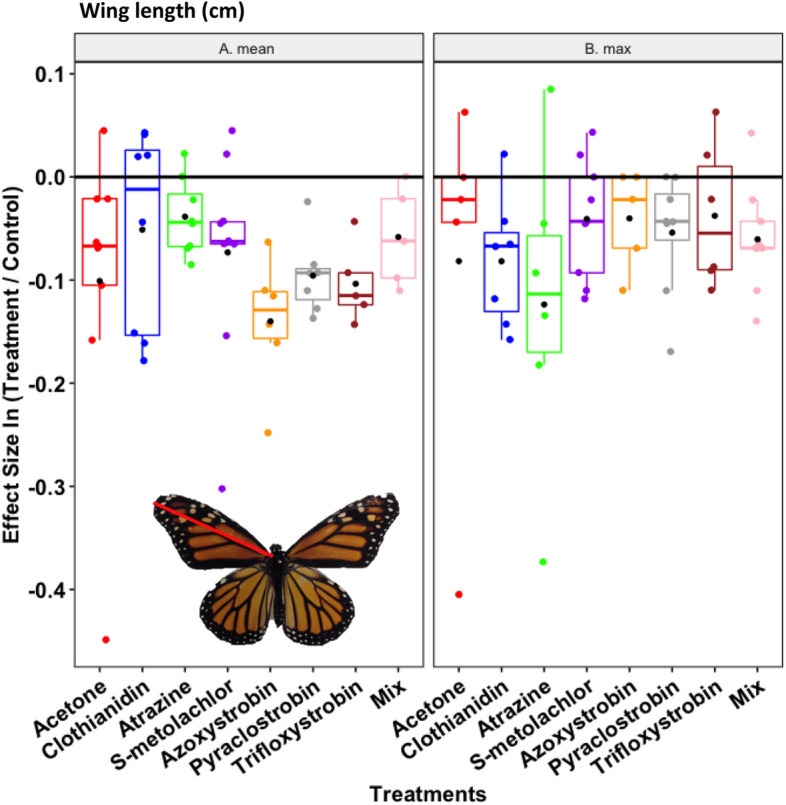
Butterfly adult size (cm wing length; red line on inset monarch photo), after being reared on milkweed leaves treated with the solvent control (acetone) and those experimentally treated to simulate the A) mean and B) maximum concentrations of field values for six pesticides and their combination (mix). An effect size at zero—i.e., the black horizontal line—represents no quantitative difference between the treatment and control, whereas positive or negative values indicate relative increases or decreases, respectively. Box plots show median values with 95% confidence intervals. Black circles are the treatment mean and colored circles are individual data points. Photo credit: Paola Olaya-Arenas.
Discussion
Isolating and experimentally testing the impact of each hypothesized factor contributing to monarch decline is critical for establishing a robust and scientifically grounded restoration plan. Although the potential threat from dietary exposure to pesticides is widely acknowledged, our experiment represents the only life cycle evaluation to date of monarchs reared with agricultural pesticides over their complete larval development on their primary host-plant, A. syriaca. As outlined in the below sections, monarch responses varied depending on developmental stage, pesticide type, and concentration. Independent of these factors, however, we found little evidence for synergism or other forms of non-additivity in the mixed pesticide ‘cocktail’ treatment. This outcome stands in contrast to studies, mainly involving honey bees, that report synergistic reactions to co-exposure of fungicides and neonicotinoids, among other combinations of active ingredients (but see68,69). Thus, we primarily considered the main effect of individual pesticide residues rather than interactions between the different components, even though they frequently co-occur on the same plant or leaf in the field52.
It is also important to consider the advantages and limitations of our experimental design. To precisely simulate field values, we used leaf cores where specific pesticide quantities that match target concentrations could be applied. This level of precision comes with a few drawbacks. For example, leaf cores sever the milkweed laticifers that deliver anti-herbivore latex to feeding sites, thereby limiting the role of plant defense compared with intact leaves. Similarly, we applied pesticides to the leaf surface and therefore cannot definitively conclude whether exposure was dietary or contact. For systemic compounds, pesticides could be transported internally from the soil through the roots rather than aerially deposited on the leaf surface, although pesticide exposure routes for milkweed are unknown. A recent study47 applied clothianidin to the soil of potted milkweed plants to effectively generate a wide spectrum of leaf concentrations, demonstrating that this approach is methodologically feasible for certain compounds.
The role of neonicotinoids in monarch decline
Based largely on an earlier study46 reporting sublethal effects of clothianidin on monarch larvae at levels as low as 1 ng/g with an LC50 of 15.63 ng/g, we expected to find strong deleterious effects of this insecticide on survival and development at the two concentrations tested (15.28 and 56.55 ng/g). This was especially the case since prior work used a short-term (36 h) pulsed bioassay, whereas we employed a longer-term continuous exposure, which should result in a correspondingly stronger impact. However, clothianidin had relatively weak effects at both concentrations for all variables measured, with a moderate and only marginally significant reduction in survival at the maximum concentration tested. While our statistical power to tease apart relatively minor differences in mortality was low, this outcome corresponds closely with data reported by Bargar et al.47 who calculated clothianidin LC50 values ranging from 47 to 205 ng/g for monarchs experiencing chronic exposure over their entire larval period, and Krishnan et al.45 who reported a range of 800–7,800 ng/g for acute exposure across instars. Given that 56.55 ng/g was the maximum value we ever recorded in > 1,000 milkweed leaf samples at multiple sites over 2 years52 and other studies reported even lower maximum concentrations (4.02 ng/g in field samples in South Dakota46), we consider it unlikely that clothianidin is a major driver of monarch declines.
One possible reason for their physiological tolerance of clothianidin is that monarchs are selected for enhanced detoxification of xenobiotics due to evolving as a specialist on a highly toxic host-plant70. Milkweeds are rich in cardenolides and other secondary metabolites, placing strong selective pressure on their herbivores71,72. This co-evolutionary process could make them predisposed to detoxifying pesticides; indeed, the pre-adaptation hypothesis predicts that host-plant chemical defenses prime an insect lineage to evolve insecticide resistance, a pattern supported in a review of published data73. It remains unclear whether cardenolide tolerance confers cross-resistance to clothianidin. However, a direct comparison between LD50 values concluded that specialist monarchs are less sensitive than generalist honey bees for most insecticide modes of action evaluated, including neonicotinoids45. Thus, using honey bees as a proxy for how all pollinators, and invertebrates in general, respond to clothianidin is probably misguided. Larval survival for another oligophagous butterfly, Polyommatus icarus (Lep.: Lycaenidae), was also robust to wide variation in foliar clothianidin levels (5–500 ng/g) on wild, leguminous host-plants74. These data suggest that clothianidin tolerance is not unique to monarchs but could be a trait that is phylogenetically conserved in specialized Lepidoptera.
Beyond their physiological response, our previously collected field data further indicate that monarch exposure to clothianidin is unlikely based on phenological asynchrony between pesticide and caterpillar52. We mainly detected clothianidin in leaves in June, before monarchs widely colonize milkweeds in our area. Yet, it is important to note that in the second year of the study we detected far more thiamethoxam on milkweed throughout the summer. Thiamethoxam is a biochemical precursor of clothianidin and widely used as a neonicotinoid seed treatment in the Midwestern US region. Although we did not directly test this compound, its toxicity to monarchs is comparable to clothianidin and several orders of magnitude less than more potent insecticides like beta‐cyfluthrin (pyrethroid) and chlorantraniliprole (anthranilic diamide)45. A recent field survey of pesticide contamination in western US milkweeds similarly concluded that chlorantraniliprole was a far greater threat to monarchs than neonicotinoids75.
As a caveat, the above conclusions are specific to wild milkweeds growing in agricultural areas. Neonicotinoids on milkweed in urban gardens can reach excessively high levels when treated as ornamentals, resulting in 100% monarch larval mortality44. Also, our assessment is specific to dietary exposure via leaf herbivory. A recent study30 found that adult monarch survival was lower when ingesting imidacloprid (23.5 ng/g) in their nectar diet; however, this outcome stands in contrast with Krischik et al.44 who reported no impact of imidacloprid at similar or higher concentrations on monarch adult survival and fecundity.
Delayed effects of larval pesticide exposure on adult performance and potential consequences for migration
We expected the immediate impact of pesticides on larval survival and growth rate to be stronger than effects expressed on later life stages. Yet, the most consistent responses in the dataset were observed in adults with longevity and wing length. This outcome is not without precedent. The butterfly Pieris brassicae was reared on cabbage plants varying in the neonicotinoid imidacloprid (0–200 ppb) with a similar outcome; larval digestion and behavior were unaffected, but adult wing size was reduced34. As with the current study (see Fig. 4A vs. B), this prior work also found that negative effects on adult traits were relatively insensitive to wide variation in concentration beyond the untreated control. Other studies testing monarch reaction to pesticides similarly show poor correspondence between dose and response46,47, even though a linear relationship is often assumed. The range of values chosen in our study, while seemingly high (i.e., the max. treatments were ca. 4- to 50-times higher than the mean treatments), were far less than many ecotoxicology studies that vary dose by 3–4 orders of magnitude and still do not detect a clear linear pattern. However, it is also challenging to interpret the effects of dose, particularly for sublethal responses, when data are only collected on survivors and higher concentrations reduce survival.
Importantly, independent of dose, we found consistent reductions in adult wing size when larvae were reared on certain pesticide treatments. Assuming that wing size is positively correlated with migratory ability, which it appears to be in monarchs76–79, these data imply that delayed effects of oral exposure as larvae could be linked with successful fall migration back to overwintering grounds in Mexico. Indeed, this possibility was suggested by Inamine et al.80 who speculated that: “the condition of fall migrants might be affected by the environments they experience early in life, including milkweed shortage, insecticides, or other changes in habitat quality”. To our knowledge, ours is the first study to substantiate this possibility, showing that oral pesticide exposure as a caterpillar leads to smaller monarch butterflies. It is critically important for future studies to take this result one step further and assess migratory behavior using flight mills or related techniques to connect pesticide ingestion, wing size, and flight capability.
One particularly interesting, but counterintuitive, finding was that monarchs lived longer when exposed to low levels of fungicides and this benefit was consistent across the three strobilurin compounds tested. While we do not have mechanistic data to support this hypothesis, we speculate that it could be a result of microbial interactions between monarchs and their environment. Our personal observations from initial monarch rearing in the laboratory indicate that this species is highly sensitive to microbial pathogens. In fact, most monarchs did not survive larval development unless their containers were sterilized daily. It should also be noted that adults were checked for the protozoan parasite Ophryocystis elektroscirrha (OE), which was never observed. We suspect that fungicides on the surface of milkweed leaves could be shifting the microbiome to a community that ultimately benefits monarchs in some fashion. This potential mechanism should be investigated in the future. It is unclear if microbial influences are unique to a laboratory environment; wild monarchs may be exposed to fewer pathogenic microorganisms due to UV exposure or other factors that reduce pathogen pressure in the field.
While adult performance metrics such as size and longevity were separately considered, co-variation in these traits could lead to life history syndromes that collectively shape the ecology of monarch butterflies. For example, fungicide exposure resulted in smaller individuals that lived longer; indeed, there was some evidence for a negative correlation between wing length and adult longevity (Supplementary Fig. 1). Since higher values of body size and lifespan are both assumed to confer a fitness benefit in insects81,82, presumably either life history combination could be successful, depending on factors such as the seasonal generation (e.g., migratory vs. resident), landscape context (e.g., density and distribution of milkweed patches), or degree of mate competition. It should also be noted that, while adult size is generally accepted as an accurate biological reflection of the quality of the larval growth environment, numerous factors impact adult longevity83. We tried to control for as many of these variables as possible, but we suspect that the duration of butterfly survival in captivity is at least partly determined by environmental features like mating status, density, sex ratio, cage area, and nectar diet. Without egg data, we cannot extend these tradeoffs to fecundity; however, female size is positively correlated with egg number in monarchs84. The only other two correlated life history traits in our dataset were: herbivory was positively associated with larval development time and pupal weight was positively associated with wing length. Both relationships are expected, given what is known about lepidopteran growth and development across life stages.
Conclusions
Overall, our data point to several key messages. First, based on the results from this experiment combined with several other recently published studies45,47,52,75, it seems unlikely that neonicotinoids are one of the primary drivers of monarch declines. This is not to say that neonicotinoids are completely innocuous, but compared with other ongoing threats (e.g., loss of milkweed host-plants and overwintering habitat), they likely play a secondary role. A continental-scale analysis that statistically teased apart the relative importance of multiple anthropogenic factors implicated in the decline ultimately came to the same conclusion40. However, more data are clearly needed on responses by adult butterflies to variation in exposure via nectar, understudied variables like fecundity, and sublethal effects on ecologically-relevant behaviors (e.g., mating, oviposition, migration). This conclusion has no bearing on the well-documented threat that neonicotinoids pose to bees and other invertebrates85.
While neonicotinoids have received the lion’s share of attention, field surveys of pesticides residues on milkweed reveal a diversity of active ingredients that warrant additional toxicity testing for monarchs52,75. Our data specifically point to fungicides as a group that should be investigated more in the future. Last, it is essential to consider the delayed, sublethal effects that are expressed on adult performance traits. Unfortunately, these are the most time consuming and challenging to measure but most directly linked with monarch fitness and migratory success. Pesticide risk assessment needs to consider these effects on adult parameters, particularly for migratory species, whenever possible.
Supplementary information
Acknowledgements
This project was produced with support of the Department of the Interior, U.S. Fish & Wildlife Service (Award no.: F16AC01256) and the Purdue AgSEED program. We thank Anurag Agrawal and Karen Oberhauser for advice on rearing monarchs and growing milkweeds; Megan McCarty for help in measuring leaf herbivory; Jacobus DeRoode and Leiling Tao for providing monarch eggs; Danielle Vasquez for statistical advice; and Ryan Drum, Sarah Warner, and Lisa Williams (USFWS) for input on the experimental design.
Author contributions
I.K. and P.O.A. designed the experiment. P.O.A. and M.E.S. developed the methodology. P.O.A. and K.H. performed the experiments. P.O.A. conducted the statistical analyses and created figures. All authors contributed to the writing of the manuscript and approved the submitted version.
Data availability
All data from this study are publicly available via the Purdue University Research Repository (PURR) at: Olaya-Arenas et al. Olaya-Arenas, P. et al. (2020), “Datasets describing monarch immature and adult performance when reared on milkweed host-plants varying in pesticides” (DOI: 10.4231/K9FH-7053).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-71211-7.
References
- 1.Potts SG, et al. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010;25:345–353. doi: 10.1016/j.tree.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Goulson D, Nicholls E, Botías C, Rotheray EL. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science. 2015;347:1255957. doi: 10.1126/science.1255957. [DOI] [PubMed] [Google Scholar]
- 3.Ollerton J. Pollinator diversity: Distribution, ecological function, and conservation. Ann. Rev. Ecol. Evol. Syst. 2017;48:353–376. [Google Scholar]
- 4.Botías C, et al. Neonicotinoid residues in wildflowers, a potential route of chronic exposure for bees. Environ. Sci. Technol. 2015;49:12731–12740. doi: 10.1021/acs.est.5b03459. [DOI] [PubMed] [Google Scholar]
- 5.Botías C, David A, Hill EM, Goulson D. Contamination of wild plants near neonicotinoid seed-treated crops, and implications for non-target insects. Sci. Tot. Environ. 2016;566:269–278. doi: 10.1016/j.scitotenv.2016.05.065. [DOI] [PubMed] [Google Scholar]
- 6.Long EY, Krupke CH. Non-cultivated plants present a season-long route of pesticide exposure for honey bees. Nat. Commun. 2016;7:11629. doi: 10.1038/ncomms11629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mogren CL, Lundgren JG. Neonicotinoid-contaminated pollinator strips adjacent to cropland reduce honey bee nutritional status. Sci. Rep. 2016;6:29608. doi: 10.1038/srep29608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsvetkov N, et al. Chronic exposure to neonicotinoids reduces honey-bee health near corn crops. Science. 2017;356:1395–1397. doi: 10.1126/science.aam7470. [DOI] [PubMed] [Google Scholar]
- 9.Wood TJ, Kaplan I, Zhang Y, Szendrei Z. Honeybee dietary neonicotinoid exposure is associated with pollen collection from agricultural weeds. Proc. R. Soc. B. 2019;286:20190989. doi: 10.1098/rspb.2019.0989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douglas MR, Tooker JF. Large-scale deployment of seed treatments has driven rapid increase in use of neonicotinoid insecticides and preemptive pest management in US field crops. Environ. Sci. Technol. 2015;49:5088–5097. doi: 10.1021/es506141g. [DOI] [PubMed] [Google Scholar]
- 11.DiBartolomeis M, Kegley S, Mineau P, Radford R, Klein K. An assessment of acute insecticide toxicity loading (AITL) of chemical pesticides used on agricultural land in the United States. PLoS One. 2019;14:e0220029. doi: 10.1371/journal.pone.0220029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douglas MR, Sponsler DB, Lonsdorf EV, Grozinger CM. County-level analysis reveals a rapidly shifting landscape of insecticide hazard to honey bees (Apis mellifera) on US farmland. Sci. Rep. 2020;10:797. doi: 10.1038/s41598-019-57225-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilburn AS, et al. Are neonicotinoid insecticides driving declines of widespread butterflies? PeerJ. 2015;3:e1402. doi: 10.7717/peerj.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forister ML, et al. Increasing neonicotinoid use and the declining butterfly fauna of lowland California. Biol. Lett. 2016;12:20160475. doi: 10.1098/rsbl.2016.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wepprich T, Adrion JR, Ries L, Wiedmann J, Haddad NM. Butterfly abundance declines over 20 years of systematic monitoring in Ohio, USA. PLoS One. 2019;14:e0216270. doi: 10.1371/journal.pone.0216270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braak N, Neve R, Jones AK, Gibbs M, Breuker CJ. The effects of insecticides on butterflies: A review. Environ. Pollut. 2018;242:507–518. doi: 10.1016/j.envpol.2018.06.100. [DOI] [PubMed] [Google Scholar]
- 17.Mulé R, Sabella G, Robba L, Manachini B. Systematic review of the effects of chemical insecticides on four common butterfly families. Front. Environ. Sci. 2017;5:32. [Google Scholar]
- 18.Russell C, Schultz CB. Effects of grass-specific herbicides on butterflies: An experimental investigation to advance conservation efforts. J. Insect Conserv. 2009;14:53–63. [Google Scholar]
- 19.Bohnenblust E, Egan JF, Mortensen D, Tooker J. Direct and indirect effects of the synthetic-auxin herbicide dicamba on two lepidopteran species. Environ. Entomol. 2013;42:586–594. doi: 10.1603/EN13021. [DOI] [PubMed] [Google Scholar]
- 20.Hahn M, Geisthardt M, Bruhl CA. Effects of herbicide-treated host plants on the development of Mamestra brassicae L. caterpillars. Environ. Toxicol. Chem. 2014;33:2633–2638. doi: 10.1002/etc.2726. [DOI] [PubMed] [Google Scholar]
- 21.Stark JD, Chen XD, Johnson C. Effects of herbicides on Behr’s Metalmark butterfly, a surrogate species for the endangered butterfly, Lange’s Metalmark. Environ. Pollut. 2012;164:24–27. doi: 10.1016/j.envpol.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Eliyahu D, Applebaum SW, Rafaeli A. Moth sex-pheromone biosynthesis is inhibited by the herbicide diclofop. Pestic. Biochem. Physiol. 2003;77:75–81. [Google Scholar]
- 23.Srivastava K, Sharma S, Sharma D, Kumar R. Effect of fungicides on growth and development of Spodoptera litura. Int. J. Life Sci. Sci. Res. 2017;3:905–908. [Google Scholar]
- 24.Nicodemo D, Mingatto FE, de Carvalho A, Veiga Bizerra PF, Tavares MA, Boas Balieira KV, Bellini WC. Pyraclostrobin impairs energetic mitochondrial metabolism and productive performance of silkworm (Lepidoptera: Bombycidae) caterpillars. J. Econ. Entomol. 2018;111:1369. doi: 10.1093/jee/toy060. [DOI] [PubMed] [Google Scholar]
- 25.Pilling ED, Jepson PC. Synergism between EBI fungicides and a pyrethroid insecticide in the honeybee (Apis mellifera) Pestic. Sci. 1993;39:293–297. [Google Scholar]
- 26.Pilling ED, Bromley-Challenor KAC, Walker CH, Jepson PC. Mechanism of synergism between the pyrethroid insecticide λ-cyhalothrin and the imidazole fungicide prochloraz, in the honeybee (Apis mellifera L) Pestic. Biochem. Physiol. 1995;51:1–11. [Google Scholar]
- 27.Johnson RM, Ellis MD, Mullin CA, Frazier M. Pesticides and honey bee toxicity—USA. Apidologie. 2010;41:312–331. [Google Scholar]
- 28.Wade A, Lin CH, Kurkul C, Regan E, Johnson RM. Combined toxicity of insecticides and fungicides applied to California almond orchards to honey bee larvae and adults. Insects. 2019;10:20. doi: 10.3390/insects10010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lichtenstein EP, Liang TT, Anderegg BN. Synergism of insecticides by herbicides. Science. 1973;181:847–849. doi: 10.1126/science.181.4102.847. [DOI] [PubMed] [Google Scholar]
- 30.James DG. A neonicotinoid insecticide at a rate found in nectar reduces longevity but not oogenesis in monarch butterflies, Danaus plexippus (L.). (Lepidoptera: Nymphalidae) Insects. 2019;10:276. doi: 10.3390/insects10090276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sinha SN, Lakhani KH, Davis BNK. Studies on the toxicity of insecticidal drift to the first instar larvae of the large white butterfly Pieris brassicae (Lepidoptera: Pieridae) Ann. Appl. Biol. 1990;116:27–41. [Google Scholar]
- 32.Davis BNK, Lakhani KH, Yates TJ. The hazards of insecticides to butterflies of field margins. Agric. Ecosyst. Environ. 1991;36:151–161. [Google Scholar]
- 33.Çilgi T, Jepson PC. The risks posed by deltamethrin drift to hedgerow butterflies. Environ. Pollut. 1995;87:1–9. doi: 10.1016/s0269-7491(99)80001-3. [DOI] [PubMed] [Google Scholar]
- 34.Whitehorn PR, Norville G, Gilburn A, Goulson D. Larval exposure to the neonicotinoid imidacloprid impacts adult size in the farmland butterfly Pieris brassicae. PeerJ. 2018;6:e4772. doi: 10.7717/peerj.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agrawal AA, Inamine H. Mechanisms behind the monarch’s decline. Science. 2018;360:1294–1296. doi: 10.1126/science.aat5066. [DOI] [PubMed] [Google Scholar]
- 36.Belsky J, Joshi NK. Assessing role of major drivers in recent decline of monarch butterfly population in North America. Front. Environ. Sci. 2018;6:86. [Google Scholar]
- 37.Malcolm SB. Anthropogenic impacts on mortality and population viability of the monarch butterfly. Ann. Rev. Entomol. 2018;63:277–302. doi: 10.1146/annurev-ento-020117-043241. [DOI] [PubMed] [Google Scholar]
- 38.Hartzler RG. Reduction in common milkweed (Asclepias syriaca) occurrence in Iowa cropland from 1999 to 2009. Crop Prot. 2010;29:1542–1544. [Google Scholar]
- 39.Pleasants JM, Oberhauser KS. Milkweed loss in agricultural fields because of herbicide use: Effect on the monarch butterfly population. Insect Conserv. Div. 2013;6:135–144. [Google Scholar]
- 40.Thogmartin WE, et al. Monarch butterfly population decline in North America: Identifying the threatening processes. R. Soc. Open. Sci. 2017;4:170760. doi: 10.1098/rsos.170760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thogmartin WE, et al. Restoring monarch butterfly habitat in the Midwestern US: ‘all hands on deck’. Environ. Res. Lett. 2017;12:074005. [Google Scholar]
- 42.Saunders SP, Ries L, Oberhauser KS, Thogmartin WE, Zipkin EF. Local and cross-seasonal associations of climate and land use with abundance of monarch butterflies Danaus plexippus. Ecography. 2018;41:278–290. [Google Scholar]
- 43.Stenoien C, et al. Monarchs in decline: A collateral landscape-level effect of modern agriculture. Insect Sci. 2018;25:528–541. doi: 10.1111/1744-7917.12404. [DOI] [PubMed] [Google Scholar]
- 44.Krischik V, Rogers M, Gupta G, Varshney A. Soil-applied imidacloprid translocates to ornamental flowers and reduces survival of adult Coleomegilla maculata, Harmonia axyridis, and Hippodamia convergens lady beetles, and larval Danaus plexippus and Vanessa cardui butterflies. PLoS One. 2015;10:e0119133. doi: 10.1371/journal.pone.0119133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krishnan N, et al. Assessing field-scale risks of foliar insecticide applications to monarch butterfly (Danaus plexippus) larvae. Environ. Toxicol. Chem. 2020;39:923–941. doi: 10.1002/etc.4672. [DOI] [PubMed] [Google Scholar]
- 46.Pecenka JR, Lundgren JG. Non-target effects of clothianidin on monarch butterflies. Sci. Nat. 2015;102:19. doi: 10.1007/s00114-015-1270-y. [DOI] [PubMed] [Google Scholar]
- 47.Bargar TA, Hladik ML, Daniels JC. Uptake and toxicity of clothianidin to monarch butterflies from milkweed consumption. PeerJ. 2020;8:e8669. doi: 10.7717/peerj.8669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hartzler RG, Buhler DD. Occurrence of common milkweed (Asclepias syriaca) in cropland and adjacent areas. Crop Prot. 2000;19:363–366. [Google Scholar]
- 49.Zaya DN, Pearse IS, Spyreas G. Long-term trends in midwestern milkweed abundances and their relevance to monarch butterfly declines. Bioscience. 2017;67:343–356. [Google Scholar]
- 50.Agrawal AA, Fishbein M. Plant defense syndromes. Ecology. 2006;87:S132–S149. doi: 10.1890/0012-9658(2006)87[132:pds]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 51.Lefevre T, Oliver L, Hunter MD, de Roode JC. Evidence for trans-generational medication in nature. Ecol. Lett. 2010;13:1485–1493. doi: 10.1111/j.1461-0248.2010.01537.x. [DOI] [PubMed] [Google Scholar]
- 52.Olaya-Arenas P, Kaplan I. Quantifying pesticide exposure risk for monarch caterpillars on milkweeds bordering agricultural land. Front. Ecol. Evol. 2019;7:223. [Google Scholar]
- 53.Olaya-Arenas P, Scharf ME, Kaplan I. Do pollinators prefer pesticide-free plants? An experimental test with monarchs and milkweeds. J. Appl. Ecol. 2020;20:20. [Google Scholar]
- 54.Bauerfeind SS, Fischer K, Hartstein S, Janowitz S, Martin-Creuzburg D. Effects of adult nutrition on female reproduction in a fruit-feeding butterfly: The role of fruit decay and dietary lipids. J. Insect Physiol. 2017;53:964–973. doi: 10.1016/j.jinsphys.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 55.Geister TL, Lorenz MW, Hoffmann KH, Fischer K. Adult nutrition and butterfly fitness: Effects of diet quality on reproductive output, egg composition, and egg hatching success. Front. Zool. 2008;5:10. doi: 10.1186/1742-9994-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Hook T, Williams EH, Brower LP, Borkin S, Hein J. A standardized protocol for ruler-based measurement of wing length in monarch butterflies, Danaus plexippus L. (Nymphalidae, Danainae) Trop. Lep. Res. 2012;22:42–52. [Google Scholar]
- 57.Davis AK, Holden MT. Measuring intraspecific variation in flight-related morphology of monarch butterflies (Danaus plexippus): Which sex has the best flying gear? J. Insects. 2015;20:591705. [Google Scholar]
- 58.García-Barros E. Multivariate indices as estimates of dry body weight for comparative study of body size in Lepidoptera. Nota Lepi. 2015;38:59–74. [Google Scholar]
- 59.Wiesweg, M. Survival Analysis: High-Level Interface for Survival Analysis and Associated Plots. R Package Version 0.1.1. (2019). https://CRAN.R-project.org/package=survivalAnalysis.
- 60.Wickham, et al. Welcome to the tidyverse. J. Open Source Softw. 2019;4:1686. [Google Scholar]
- 61.Kassambara, A. ggpubr: ‘ggplot2’ Based Publication Ready Plots. R Package Version 0.2.5. (2020). https://CRAN.R-project.org/package=ggpubr.
- 62.Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. R package version 0.4.0. (2020). https://CRAN.R-project.org/package=rstatix.
- 63.Fox J, Weisberg S. An R Companion to Applied Regression. 3. Thousand Oaks: Sage; 2019. [Google Scholar]
- 64.Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag; 2016. [Google Scholar]
- 65.Kassambara, A. ggcorrplot: Visualization of a Correlation Matrix Using ‘ggplot2’. R Package Version 0.1.3. (2019) https://CRAN.R-project.org/package=ggcorrplot.
- 66.Hedges LV, Gurevitch J, Curtis PS. The meta-analysis of response ratios in experimental ecology. Ecology. 1999;80:1150–1156. [Google Scholar]
- 67.Schaarschmidt, F. & Gerhard, D. pairwiseCI: Confidence Intervals for Two-Sample Comparisons. R Package Version 0.1-27. (2019) https://CRAN.R-project.org/package=pairwiseCI.
- 68.Thompson HM, Fryday SL, Harkin S, Milner S. Potential impacts of synergism in honeybees (Apis mellifera) of exposure to neonicotinoids and sprayed fungicides in crops. Apidologie. 2014;45:545–553. [Google Scholar]
- 69.Tadei R, et al. Late effect of larval co-exposure to the insecticide clothianidin and fungicide pyraclostrobin in Africanized Apis mellifera. Sci. Rep. 2019;9:3277. doi: 10.1038/s41598-019-39383-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Després L, David JP, Gallet C. The evolutionary ecology of insect resistance to plant chemicals. Trends Ecol. Evol. 2007;22:298–307. doi: 10.1016/j.tree.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 71.Jones PL, Petschenka G, Flacht L, Agrawal AA. Cardenolide intake, sequestration, and excretion by the monarch butterfly along gradients of plant toxicity and larval ontogeny. J. Chem. Ecol. 2019;45:264–277. doi: 10.1007/s10886-019-01055-7. [DOI] [PubMed] [Google Scholar]
- 72.Karageorgi M, et al. Genome editing retraces the evolution of toxin resistance in the monarch butterfly. Nature. 2019;574:409–412. doi: 10.1038/s41586-019-1610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hardy NB, Peterson DA, Ross L, Rosenheim JA. Does a plant-eating insect’s diet govern the evolution of insecticide resistance? Comparative tests of the pre-adaptation hypothesis. Evol. Appl. 2018;11:739–747. doi: 10.1111/eva.12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Basley K, Goulson D. Effects of field-relevant concentrations of clothianidin on larval development of the butterfly Polyommatus icarus (Lepidoptera, Lycaenidae) Environ. Sci. Technol. 2018;52:3990–3996. doi: 10.1021/acs.est.8b00609. [DOI] [PubMed] [Google Scholar]
- 75.Halsch C, et al. Pesticide contamination of milkweeds across the agricultural, urban, and open spaces of low elevation Northern California. Front. Ecol. Evol. 2020;8:162. [Google Scholar]
- 76.Altizer S, Davis AK. Populations of monarch butterflies with different migratory behaviors show divergence in wing morphology. Evolution. 2010;64:1018–1028. doi: 10.1111/j.1558-5646.2010.00946.x. [DOI] [PubMed] [Google Scholar]
- 77.Dockx C. Directional and stabilizing selection on wing size and shape in migrant and resident monarch butterflies, Danaus plexippus (L.) Cuba. Biol. J. Linn. Soc. 2007;92:605–616. [Google Scholar]
- 78.Satterfield DA, Davis AK. Variation in wing characteristics of monarch butterflies during migration: Earlier migrants have redder and more elongated wings. Anim. Migr. 2014;2:1–7. [Google Scholar]
- 79.Freedman MG, Dingle H. Wing morphology in migratory North American monarchs: Characterizing sources of variation and understanding changes through time. Anim. Migr. 2018;5:61–73. [Google Scholar]
- 80.Inamine H, Ellner SP, Springer JP, Agrawal AA. Linking the continental migratory cycle of the monarch butterfly to understand its population decline. Oikos. 2016;125:1081–1091. [Google Scholar]
- 81.Nylin S, Gotthard K. Plasticity in life-history traits. Ann. Rev. Entomol. 1998;43:63–83. doi: 10.1146/annurev.ento.43.1.63. [DOI] [PubMed] [Google Scholar]
- 82.Awmack CS, Leather SR. Host plant quality and fecundity in herbivorous insects. Ann. Rev. Entomol. 2002;47:817–844. doi: 10.1146/annurev.ento.47.091201.145300. [DOI] [PubMed] [Google Scholar]
- 83.Jervis MA, Boggs CL, Ferns PN. Egg maturation strategy and its associated trade-offs: A synthesis focusing on Lepidoptera. Ecol. Entomol. 2005;30:359–375. [Google Scholar]
- 84.Oberhauser KS. Fecundity, lifespan and egg mass in butterflies: Effects of male-derived nutrients and female size. Funct. Ecol. 1997;11:166–175. [Google Scholar]
- 85.Main AR, Webb EB, Goyne KW, Mengel D. Neonicotinoid insecticides negatively affect performance measures of non-target terrestrial arthropods: A meta-analysis. Ecol. Appl. 2018;28:1232–1244. doi: 10.1002/eap.1723. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data from this study are publicly available via the Purdue University Research Repository (PURR) at: Olaya-Arenas et al. Olaya-Arenas, P. et al. (2020), “Datasets describing monarch immature and adult performance when reared on milkweed host-plants varying in pesticides” (DOI: 10.4231/K9FH-7053).