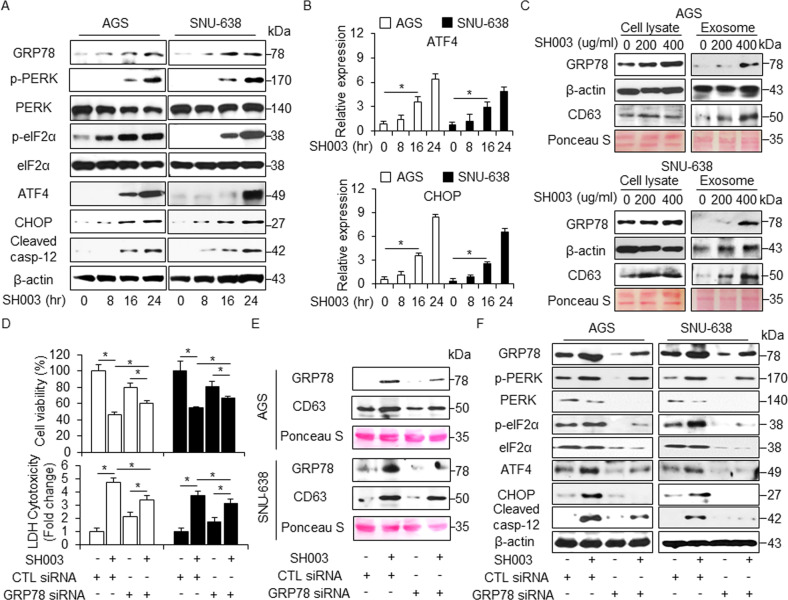
Fig. 5. SH003 induces ER stress response in GC cells.
a AGS and SNU-638 cells were treated with SH003 (400 μg/mL) for indicated times and ER stress markers including GRP78, p-PERK, PERK, p-eIF2α, eIF2α, ATF4, CHOP, and cleaved caspase-12 were assessed by Western blot assay. b AGS and SNU-638 cells were treated with SH003 (400 μg/mL) for the indicated times and ER stress markers, including ATF4 and CHOP, were assessed by real-time RT-PCR. c AGS and SNU-638 cells were treated with SH003 in a dose-dependent manner (0, 200, and 400 μg/mL, 24 h) and exosomes were collected from the supernatant of the cells. Protein purification for exosomes and cell lysates was quantified by Ponceau S staining. These samples were also examined by Western blotting using the exosome marker, CD63 and the ER stress marker, GRP78. d AGS and SNU-638 cells were transfected with control or GRP78 siRNA in the presence or absence of SH003 (400 μg/mL, 24 h), and then, cell viability and LDH assay were performed; *p < 0.05. e Western blot analysis of GRP78 and CD63 in exosomes isolated from SH003 (400 μg/mL, 24 h)-treated AGS and SNU-638 cell culture media in the presence or absence of GRP78 siRNA (30 nM, 24 h).). β-actin and Ponceau S were used as protein loading controls. f Western blot analysis of GRP78, p-PERK, p-eIF2α, ATF4, CHOP, and cleaved caspase-12 in SH003 (400 μg/mL, 24 h)-treated AGS and SNU-638 cells in the presence or absence of GRP78 siRNA (30 nM, 24 h). β-actin was used as protein loading controls.