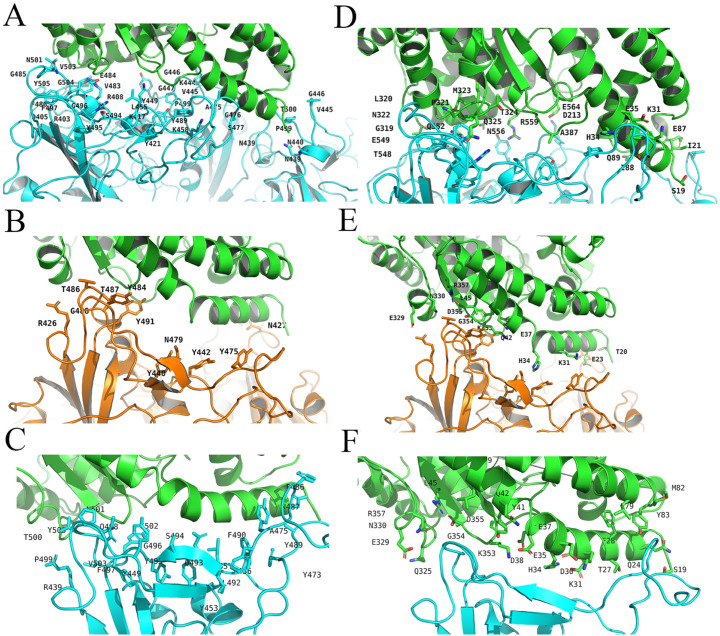
Fig. 2.
ACE2 Residues in full-length SARS-CoV2 S protein (A), full-length SARS-Cov S protein (B) and RBD structure of SARS-Cov 2 (C) were present. S protein residues in full-length SARS-CoV2 S protein (D), full-length SARS-Cov S protein (E) and RBD structure of SARS-Cov 2 (F) were present as well. Human ACE2 protein was marked in green. SARS-CoV2 S protein was marked in cyan and SARS-Cov S protein was marked in orange. In the result of RBD model 6VW1, the 100 ns MD structure reviewed ACE2 residues H34, Y41, Q42, D30, K31, K353, R357, E329, Q325 were involved in the interaction, while the full-length model suggested not only ACE2 residues K31, H34, Q325 but also residue E564, N556, R559 in ACE2 might mediate the interaction. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)