Table 1.
Optimization Studiesa
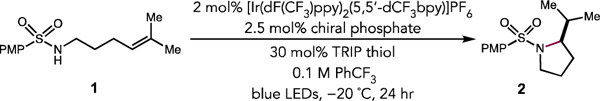 | |||
|---|---|---|---|
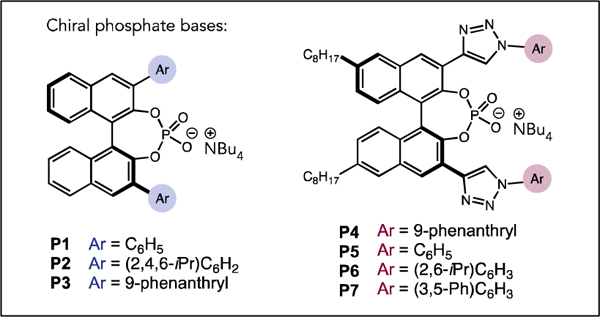
| Entry | Phosphate | Yield (%) | er |
| 1 | P1 | 54 | 55:45 |
| 2 | P2 | 30 | 52:48 |
| 3 | P3 | 96 | 35:65 |
| 4 | P4 | 62 | 86:14 |
| 5 | P5 | 97 | 93:7 |
| 6 | P6 | 98 | 87:13 |
| 7 | P7 | 85 | 95:5 |
 | |||
| Entry | Change from Entry 7 | Yield (%) | er |
| 8 | room temperature | 93 | 89:11 |
| 9 | thiophrenol H-atom donor | 81 | 94:6 |
| 10 | 10 mol% P7 | 89 | 95:5 |
| 11 | no base | <1 | - |
| 12 | no thiol | <5 | - |
| 13 | no photocatalyst | <1 | - |
| 14 | no light | <1 | - |
Reactions were conducted on a 0.05 mmol scale, and yields determined by NMR analysis relative to an internal standard. Enantioselectivity was determined by HPLC analysis on a chiral stationary phase.
