Table 2.
Scope of Enantioselective Amination Reactiona
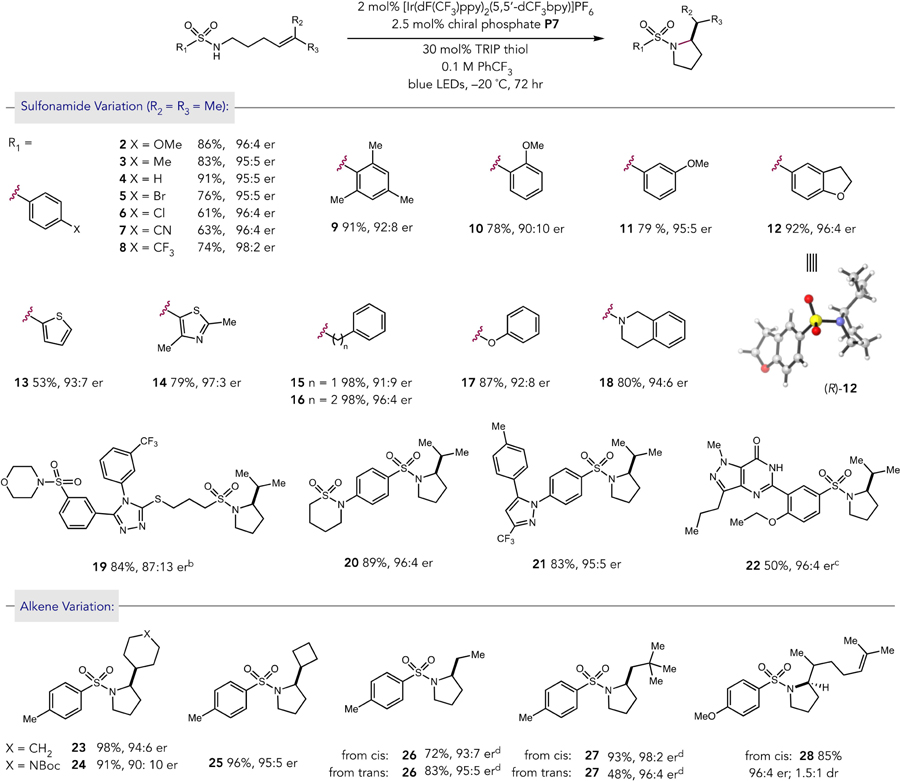 |
Yields and enantioselectivities are for isolated material following chromatography on silica gel and are the average of two experiments. Reactions were conducted on 0.5 mmol scale.
Reaction was run at room temperature.
Reaction was run in dichloromethane.
Reactions were run at 0 °C with substitution of TRIP-disulfide for thiol.
