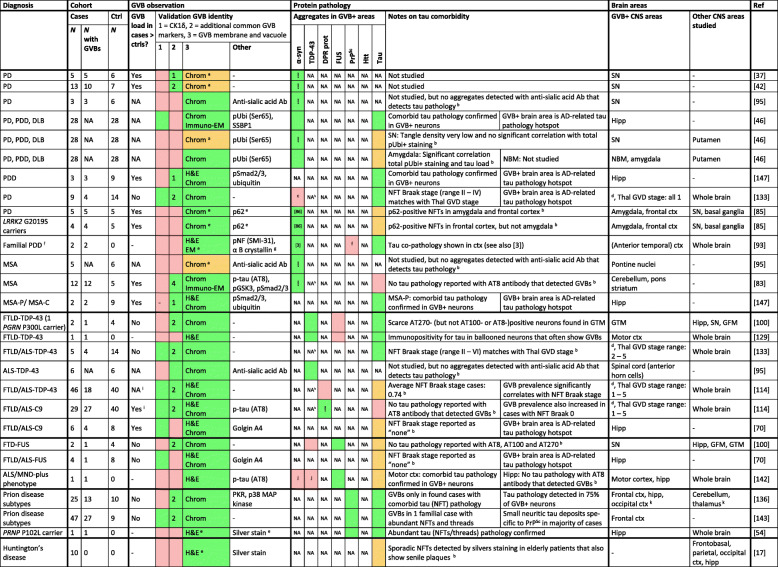
Table 4.
Putative GVB observations and comorbid tau pathology in “non-tau” neurodegenerative proteinopathies
Diagnosis: clinical diagnosis and if applicable causative mutations are listed. Cohort: the number (N) of cases, of cases in which GVBs are detected and of non-neurological controls are listed. GVB observation: if a control group was included, it is indicated whether the GVB load was higher in cases than in controls. Color-coding is used to denote if studies meet the criteria for validation of GVB identity (Table 1; Fig. 1). Green: criterion met; red: criterion not met; orange: see a. For criterion 2, the number of additional common GVB markers used in the study is noted. For criterion 3, the method used is indicated. Other antibodies and methods used to probe GVBs are listed, with antibody names or phospho-epitopes shown in brackets. Protein pathology: Color-coding is used to denote the presence of protein aggregates in those brain areas where GVBs were detected. Green: detected (reference to publications showing the presence of these protein aggregates is included if it was not shown in the same study); red: not detected; orange: possible tau comorbidity, see additional notes hereon in the next column; NA: not available (either this protein was not studied or no data is available on the presence of this protein specifically in the GVB-positive brain areas); ! co-localization of non-tau protein aggregates and GVBs in the same cells. Additional notes on the detection of tau pathology in GVB-containing brain areas are listed. For NFT Braak staging see [13]. CNS areas: CNS areas where GVBs were and were not reported are listed. Reference to publications are included (Ref). a In these studies, GVB-like structures were described as smaller granules/punctate structures as the GVB vacuole and membrane did not stand out upon chromogenic detection. Notably, other chromogens than DAB were used given the presence of neuromelanin in studied neurons. b Comorbid tau pathology was not conclusively excluded in GVB-containing cells. c Probably absent, based on a PD Braak stage of 4-5. For PD Braak staging see [14]. d Thal GVD staging is performed and the range of Thal GVD stages is reported for those cases with GVBs. For brain areas affected by GVBs in each Thal GVD stage see [132]. e No typical example of putative GVBs shown (in case of Huntington’s disease because no GVBs were found). f A later report stated that this was probably the SNCA G51D mutation [62]/PrPSc pathology was excluded in 1 of 2 cases. g Putative GVBs were visible due to strong staining of the whole cytoplasm rather than GVB-specific staining. h pTDP-43 antibody was used to detect GVBs and no pTDP-43-positive inclusions were reported in GVB-bearing cells. i FTLD/ALS-C9, but not FTLD/ALS-nonC9, cases were directly compared to controls. j Comorbid α-synuclein and TDP-43 pathology excluded in motor cortex, but not hippocampus. k Occipital cortex, cerebellum and thalamus were only studied for specific prion disease subtypes. A single positive neuron was detected in the occipital cortex of one case. Note that for clarity Aβ comorbidity is not included in this table
Abbreviations: AD Alzheimer’s disease, ALS amyotrophic lateral sclerosis, Chrom GVB morphology visualized by chromogenic peroxidase-catalyzed immunodetection of GVB core marker, CNS central nervous system, Ctrl controls, Ctx cortex, DLB Dementia with Lewy bodies, DPR prot dipeptide repeat proteins, FTLD frontotemporal lobar degeneration, FTLD/ALS-C9 TDP-43 related FTLD/ALS caused by a hexanucleotide repeat expansion in C9ORF72, FTLD-FUS FUS-related FTLD, FTLD/ALS-FUS FUS-related FTLD/ALS, FTLD-TDP-43 TDP-43-related FTLD, FTLD/ALS-TDP-43 TDP-43-related FTLD/ALS, GFM gyrus frontalis medialis, GTM gyrus temporalis medialis, GVB granulovacuolar degeneration body, GVB+ GVB-positive/-containing, GVD granulovacuolar degeneration, H&E hematoxylin and eosin, Hipp Hippocampus, Immuno-EM electron microscopy with immunodetection of GVB marker, LB Lewy bodies, MND motor neuron disease, MSA multiple system atrophy, MSA-C MSA with cerebellar ataxia, MSA-P MSA with parkinsonism, NBM nucleus basalis of Meynert, NFT neurofibrillary tangle, p phosphorylated, PD Parkinson’s disease, PDD Parkinson’s disease with dementia / parkinsonism and dementia, Ref reference, SN substantia nigra, > higher than, - not applicable. For abbreviations of protein and gene names and antibodies see “List of abbreviations”