Abstract
Environmental monitoring through gas sensors is paramount for the safety and security of industrial workers and for ecological protection. Graphene is among the most promising materials considered for next-generation gas sensing due to its properties such as mechanical strength and flexibility, high surface-to-volume ratio, large conductivity, and low electrical noise. While gas sensors based on graphene devices have already demonstrated high sensitivity, one of the most important figures of merit, selectivity, remains a challenge. In the past few years, however, surface functionalization emerged as a potential route to achieve selectivity. This review surveys the recent advances in the fabrication and characterization of graphene and reduced graphene oxide gas sensors chemically functionalized with aromatic molecules and polymers with the goal of improving selectivity toward specific gases as well as overall sensor performance.
Introduction
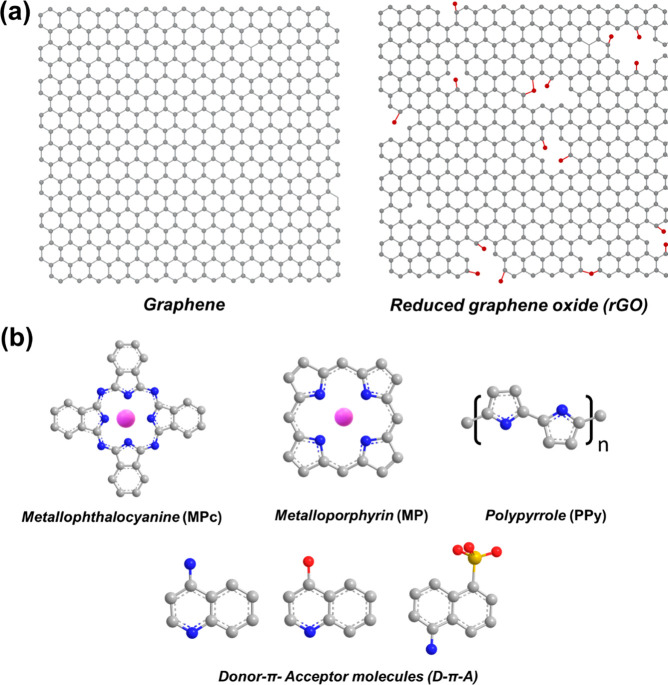
Graphene, an extended honeycomb network of sp2-hybridized carbon atoms,1 is among the strongest candidates for the development of new gas sensing technologies due to its high surface-to-volume ratio, mechanical strength and flexibility, large conductivity, and low electrical noise.2−8 Methods of graphene synthesis include both top-down approaches (mechanical exfoliation from graphite) and bottom-up large-area approaches such as epitaxial growth or chemical vapor deposition (CVD). An alternative route toward large-scale production of graphene-like materials is the reduction of graphene oxide (GO) to reduced graphene oxide (rGO). rGO, as schematically presented in Figure 1a, has a graphene-like basal plane that is decorated with structural defects during its synthesis and occupied with areas containing oxidizing chemical groups.9
Figure 1.
(a) Lattice structure of graphene and reduced graphene oxide. (b) Molecules used to functionalize graphene and rGO for sensing applications.
While gas sensors based on graphene devices have already demonstrated high sensitivity, one of the most important figures of merit, selectivity, remains a challenge.
Selectivity is the ability of a gas sensor to have higher sensitivity to a specific target analyte when exposed to multiple interfering gases. This implies that the sensor’s response to the target gas should be higher than the response to interfering gases. In the case of graphene chemiresistors, the sensing mechanism relies on changes in graphene’s conductivity upon exposure to gaseous species. Generally, different gases with electron-withdrawing or electron-donating molecules could be adsorbed on graphene’s surface and change its conductivity. Such a sensing platform has intrinsically high sensitivity, as graphene’s conical band structure ensures significant conductivity changes. At the same time, selectivity can be a challenge for a sensitive chemiresistor, as many gases can lead to large sensing responses. Functionalization of the graphene surface has been proposed as an approach to overcome this issue. In this case, a selective detection relies on the different interaction strengths between analytes and the functional species, that can preferentially anchor a desired target gas.
In the past few years, experimental advances demonstrated that surface functionalization10 is indeed a promising route to achieve selectivity. Adsorbates ranging from metal oxides, metal nanoparticles, polymers, and organometallic molecules have been successfully used to improve figures of merit such as sensitivity, recovery time, and especially selectivity. In this Mini-Review, we focus on recent research exploring graphene molecular functionalization for improved sensor selectivity.
Graphene molecular functionalization can be accomplished both covalently and noncovalently. To form covalent bonds between graphene and a target molecule, several chemical transformations such as nucleophilic, cycloaddition, condensation, and electrophilic reactions are used. In this case, graphene’s π-orbitals are involved in the bond, transforming sp2 into sp3 bonds. This change in the hybridization of graphene alters its electronic transport properties. On the other hand, noncovalent functionalization offers the opportunity of attaching functional groups to the graphene surface based on electrostatic interactions as van der Waals forces or π–π interactions. Aromatic molecules with planar structures are ideal candidates for this purpose, since they can be easily anchored to graphene surface through π–π interactions. Such noncovalent interactions do not alter the conjugated network of graphene, thus preserving its intrinsic transport properties.
Both covalent and noncovalent functional modification of graphene with electron-donating or electron-withdrawing molecules can lead to charge transfer, resulting in p-doping or n-doping of the graphene film. The charge transfer mechanisms among analytes, functional molecules and graphene play a key role in determining the gas-sensing performance of devices.
A variety of graphene hybrids have been studied as sensing material alternatives with the goal of improving the performance of bare graphene gas sensors. Organic molecules such as porphyrins and phthalocyanines (Pcs) have been used to improve the selectivity of graphene-based sensors. Porphyrins are macrocycles with 18 π-electron conjugated ring system and can be transformed into metalloporphyrins (MPs) by substituting two hydrogen atoms with a transition metal atom at the central core (Figure 1b). The strong catalytic activity of MPs makes it an attractive functional material that can enhance the chemical response of graphene. Phthalocyanines are two-dimensional 18 π-electron materials with high electronic delocalization. Metallophthalocyanines (MPcs) can be formed by the substitution of hydrogen atoms with a transition metal as seen in Figure 1b. Their thermal and chemical stability, together with the highly tunable structure facilitating the design of a wide range of compounds, make MPcs promising candidates for gas sensing. Conductive polymers have also been used as a sensitive layer in graphene hybrids. Among them, polypyrrole (PPy) (Figure 1b) is a conjugated polymer that has emerged as a promising material for sensing applications due to its low cost, easy synthesis, and moderately high conductivity and wide detection of variety of volatile compounds. Moreover, its most promising feature is the ability to operate at room temperature. Donor-π-acceptor (D-π-A) systems are a class of molecules with delocalized π orbitals, in which the electron migration is facilitated from the donor to the acceptor group. These molecules usually have high chemical stability and good electronic and optical properties. D-π-A molecules have been incorporated in graphene-based hybrids for sensing, as their structural architecture can enhance the charge transfer between graphene and a target gas analyte.
This Mini-Review is focused on the latest efforts to improve graphene sensor selectivity with the introduction of organic molecules and polymers that tailor the graphene response toward specific gases. We focus both on the surface functionalization methods and characterization and on the sensing performance, as the two aspects are interconnected.
I. Hybrids Based on Graphene/Metalloporphyrins (MPs)
Porphyrins with different coordinated metals have been used for functionalizing graphene, as their aromatic backbone promotes noncovalent bonding with the graphene plane. MPs have strong dipoles when interacting with specific gas analytes, making them good candidates for improving the selectivity of graphene gas sensors.
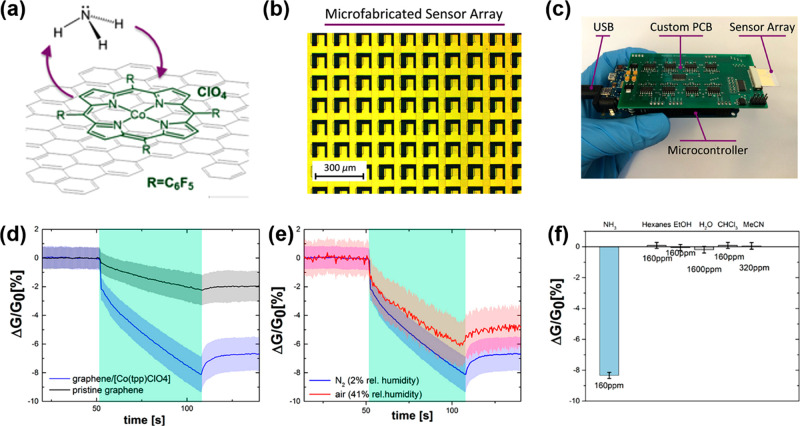
Mackin and co-workers11 reported a highly sensitive ammonia gas chemiresistor based on CVD graphene functionalized with Co porphyrin Co(tpfpp)ClO4–5,10,15,20-tetrakis(pentafluorophenyl) porphyrinato cobalt(III) perchlorate as shown in Figure 2a). The porphyrin solution was dropcasted onto a CVD-graphene sensor array. These were tested using a system especially designed to efficiently evaluate a large number of sensors (Figure 2b,c). As shown in Figure 2d, graphene functionalization increased the sensitivity of the sensors to ammonia in comparison with pristine graphene. High sensitivity to ammonia was found for the functionalized sensors when tested in the presence of interfering compounds such as dry nitrogen or air (Figure 2e). This study finds that when functionalized with Co porphyrin, the sensor response to ammonia increased four times. In terms of selectivity, as presented in Figure 2f, the sensors showed negligible response to water and four organic solvents in comparison to ammonia. Sensor-to-sensor variation was evaluated when testing the response of 160 sensors, and it was found that there are differences in their baseline currents. In order to overcome this problem, they note the need for future development of more uniform graphene growth, transfer, and microfabrication techniques that will reduce the variation in the sensing material properties.
Figure 2.
(a) Chemical structure of the Co porphyrin molecule on top of graphene. (b) Microscope image of graphene sensor array. (c) Complete measurement system and sensor array insert. (d) Mean change in conductance upon exposure to 160 ppm of NH3 in nitrogen of the pristine graphene sensor and the Co porphyrin functionalized graphene with shaded regions representing plus or minus one standard deviation from the mean. (e) Mean change in conductance of the Co porphyrin functionalized graphene sheet upon exposure to 160 ppm of NH3 in dry nitrogen and air. (f) Selectivity comparison of the functionalized graphene. Adapted with permission from ref (11). Copyright 2018, American Chemical Society. Photograph in part c adapted with permission from ref (11).
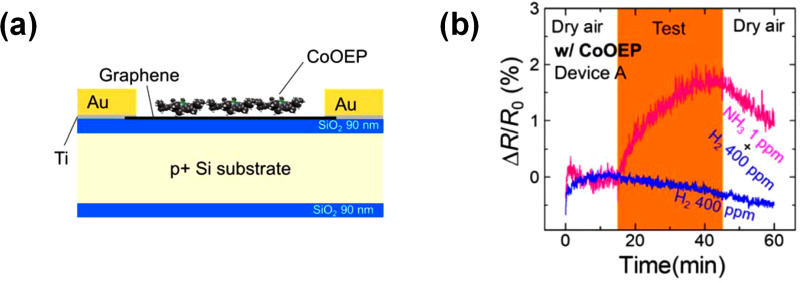
Recently, hybrid Co porphyrin (CoOEP–2,3,7,8,12,13,17,18-Octaethyl-21H,23H-porphine cobalt(II))/CVD graphene were evaluated as gas sensing materials.12 To functionalize the CVD graphene film, a saturated chloroform Co porphyrin solution was spin-coated onto a graphene sensor array (Figure 3a). Successful graphene functionalization was evidenced by the XPS measurements of the hybrids indicating characteristic features of pyrrole rings in Co porphyrin and C=C bonds in the graphene network. The performance of the hybrid Co porphyrin/CVD graphene sensors was tested under low concentrations of NH3 (2 ppm). The functionalized sensors showed a six-times greater response to ammonia when compared to non-functionalized graphene. Since H2 can prevent accurate detection of ammonia in breathalyzers, the selectivity of the sensors was evaluated for mixtures of H2 and ammonia. The functionalized sensors did not show response to high concentrations of H2 (400 ppm), but recognized low concentrations of NH3 (1 ppm), confirming that the sensor response to ammonia is not affected by the presence of H2 (Figure 3b). Moreover, it was found that the sensitivity of the sensors to ammonia was not disrupted by the presence of high humidity concentrations. The improved sensing performance of the functionalized graphene is explained as an increase in NH3 adsorption sites in comparison with non-functionalized graphene. CVD graphene functionalized with Co porphyrins was reported also as a sensing material for detecting volatile organic compounds (VOC).13 After the fabrication of the graphene channel, the Co porphyrin–5,10,15,20-tetraphenyl-21H, 23H-porphyrin cobalt(II) was deposited on the graphene by thermal evaporation. Raman spectroscopy and AFM measurements confirmed that the graphene film was a single layer. The hybrid sensors were electrically characterized in a field effect transistor (GFET) configuration. The electrical measurements revealed that sensors were n-doped, and their resistance decreased upon exposure to toluene. Moreover, functionalized graphene sensors had a higher response (>2 times) than graphene sensors, which was attributed to the formation of donor–acceptor complexes at the interface between graphene and the Co porphyrin.
Figure 3.
(a) Schematic of a CoOEP/graphene sensor.12 (b) Selectivity of CoOEP/graphene sensor for ammonia against hydrogen. Adapted with permission from ref (12). Copyright 2018, American Chemical Society.
Quasi-freestanding epitaxial graphene (QFeG) functionalized with Co porphyrin was reported as a hybrid material for ammonia sensing by Iezhokin and co-workers.14 Functionalization was achieved by drop-casting Co porphyrin heptane dispersions on top of graphene. The Co porphyrin used in this work was CoMP–5,10,15,20-meso-tetrakis-nonadecylporphyrin cobalt(II). AFM measurements, Hall Effect experiments, and Density functional theory (DFT) calculations revealed that the graphene surface is covered by a single porphyrin layer. It is reported that, while QFeG has a negligible response to ammonia, the functionalized graphene hybrid can detect NH3 below 100 ppb. After functionalization, the CoMP/QFeG sensor is p-doped. Upon NH3 adsorption on the metallic Co centers, charge transfers to the underlying QFeG. Both electrical measurements and theoretical band structure calculations support the proposed electron doping mechanism that shifts the Fermi level.
In a different study, CVD graphene was functionalized with Zn porphyrins (ZnTTPOH–5-(4-hydroxyphenyl)-10,15,20-tri(p-tolyl) zinc porphyrin) for the detection chemical explosive molecules (TNT) in the vapor phase.15 It is proposed that the ZnTTPOH molecules act as a selective spot for absorbing the TNT molecules and the electron transfer from the graphene to TNT is mediated by ZnTTPOH. The charge transfer is detected as a shift in the Dirac point by the hybrid GFETs. The selectivity of such sensors was explored against different nitroaromatic explosives (nitrobenzene, m-dinitrobenzene, nitromethane) and VOCs like isopropanol and acetone.
II. Hybrids Based on Graphene/Polypyrrole Molecules
Graphene functionalized with polymers usually presents porous structures that enhance the diffusion of the molecules to the sensing layers. Moreover, polymers can form strong bonds with target gases through hydrogen bonds or π–π stacking, improving the selectivity of the sensors.
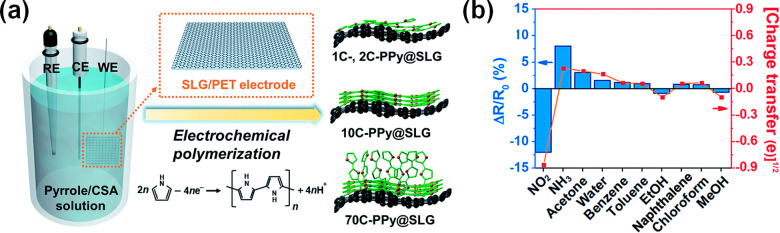
Flexible and transparent sensors based on CVD graphene functionalized with polypyrrole (PPy) were recently reported.16 Graphene functionalization was achieved by electrochemical polymerization of pyrrole monomers as described in Figure 4a. The fabricated sensors were tested for different gases, showing the best detection limits of 0.03 ppb for NO2 and 0.04 ppb for NH3 (Figure 4b). It is noted that upon exposure to NO2, the electrical resistance of the sensors decreased, while it increased when exposed to NH3. The good sensing performance of the hybrid system was attributed to the well-ordered structure of PPy/graphene, which enhances the charge mobility.
Figure 4.
(a) Schematic illustration of the synthesis of polypyrrole (PPy) on a single layer graphene/polyethylene terephthalate (SLG/PET) film: a three-electrode system (RE, reference electrode; WE, working electrode; CE, counter electrode), an electrochemical polymerization scheme, and nCycles-PPy on single layer graphene. (b) Evaluation of the sensor selectivity for various target analytes (1 ppm). Adapted with permission from ref (16). Copyright 2018, Royal Society of Chemistry.
In a similar approach, ammonia sensors were fabricated by PPy electropolymerization synthesis on CVD-grown graphene.17 In general, higher response to ammonia was shown for the PPy/graphene compared to the graphene sensor without PPy layer. The sensors were exposed to 5 ppm of NH3 at room temperature, showing good selectivity for NH3 in the presence of interfering gases CH2O and NO2. Electron transfer from NH3 to PPy and then to graphene were proposed as responsible for the increased sensitivity.
III. Hybrids Based on Reduced Graphene Oxide/Metallophthalocyanines (MPc)
Metallophthalocyanines (MPcs) typically have electron-donating properties, however, electron-accepting Pcs can also be achieved by placing appropriate substituents at its periphery. This adaptability makes them ideal molecules to tailor graphene sensors to anchor electron-withdrawing or electron-donating gases.
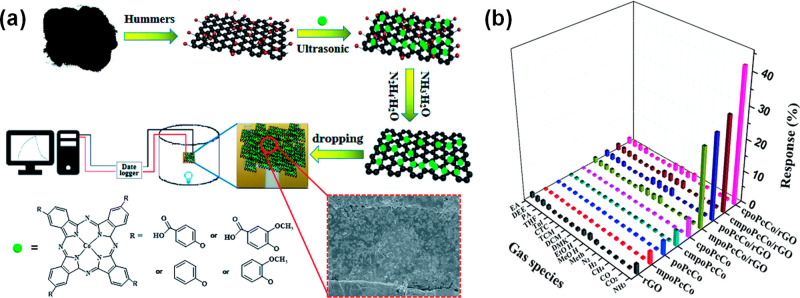
Zhou et al.18 proposed ammonia gas sensors based on rGO functionalized using Cu phthalocyanines, 3-CuPc–1,8,15,22-tetra-iso-pentyloxyphthalocyanine copper. The hybrids were prepared by hydrazine reduction of GO in the presence of 3-CuPc and were subsequently drop-casted onto interdigitated electrodes. The noncovalent attachment of the 3-CuPc molecules onto the surface of rGO was characterized by UV–vis and X-ray photoelectron spectroscopy (XPS). The results indicated that 3-CuPc molecules were successfully anchored to the surface of the rGO sheets by π–π stacking interaction. The sensor response of the hybrid materials was evaluated at different ammonia concentrations (400 ppb to 3200 ppm) and compared with pristine rGO. To assess the selectivity to ammonia of the 3-CuPc/rGO sensors, their response was measured when exposed to NH3, CO2, CH4, H2, and CO. It was found that the response to ammonia was 15 times higher than to the other gases. The mechanism responsible for the improved gas sensing performance was attributed to the charge transfer between rGO, 3-CuPc, and NH3. The electrons are transferred from the NH3 to the phthalocyanine molecule, which acts as the dominant active site for NH3 adsorption. After adsorption, the ease of charge transfer between 3-CuPc derivatives and rGO results in the relatively large sensitivity of such sensors. Guo et al.19 explored gas sensing performance of four nanohybrid materials synthesized based on rGO and Co phthalocyanines (CoPc) containing different phenoxyl substituents (cpoCoPc, poCoPc, cmpoCoPc, and mpoCoPc). After the nanohybrid synthesis, solutions of the Pcs/rGO were dropped on gold interdigitated electrodes using a microsyringe, as shown in Figure 5a. UV–vis spectroscopy found that the interaction between rGO and Pcs leads to reduction in the HOMO–LUMO energy gap of the phthalocyanines. The performance of the hybrid sensors, rGO, and Pcs films was evaluated for 18 different gases including ammonia (Figure 5b). The results revealed selectivity to ammonia in comparison to other gases for all hybrids, with a strongest response obtained for cpoCoPc/rGO. The cpoCoPc/rGO hybrid exhibited a high gas response of 42.4% for 100 ppm of NH3 with a reversible recovery time of 120 s and low detection limit of 3.7 ppb. It was proposed that the electron-withdrawing carboxyl group present on the phenoxyl substituents of the Pc ring increases the hole sites of the phthalocyanine, which are active sites for NH3 adsorption. The fast recovery time of the sensors was understood as electron loss of the hybrids when reacting with reappearing oxygen molecules in the desorption process of NH3.
Figure 5.
(a) Synthesis and processing schematic of PcCo/rGO hybrids. (b) Response of PcCos, rGO, and PcCo/rGO hybrid-based sensors to 100 ppm of NH3 and 5000 ppm of other gases. Adapted with permission from ref (19). Copyright 2019, Royal Society of Chemistry.
The effect of different substituted Pcs on the ammonia sensing performance of rGO/Pcs hybrids was evaluated.20 Three different sensors were fabricated with Co amino Pcs (ABOCoPc, ACoPc), substituent-free cobalt phthalocyanine (FCoPc), and rGO. Dispersions of the prepared hybrids in dimethylformamide (DMF) were dropped on interdigitated gold electrodes. The functionalization of rGO was confirmed with FTIR, UV–vis, and Raman spectroscopy. When tested under different ammonia concentrations, the CoPc/rGO sensors outperformed the rGO sensors in response and recovery time. In addition, the hybrid sensors were selective toward ammonia in comparison with other 16 tested gaseous compounds (VOCs and NOx). The performance of the sensors is attributed to the presence of the amino groups of the ACoPc, a strong electron-donor group, providing electrons that when combined with holes in p-type rGO weaken charge transfer between ACoPc/rGO and adsorbed gas molecules. Phthalocyanines with different metallic centers have been also explored in order to tailor the performance of graphene sensors. Li et al.21 described the fabrication of hybrid MPc/rGO sensors using an identical type of Pc core but varying the metallic center MPc (M = Ni, Cu, Pb). The fastest response time was found for NiPc/rGO and the fastest recovery time for CuPc/rGO; however, the three MPc/rGO recovered completely to the original resistance, contrary to rGO. MPc/rGO hybrid sensor was able to detect NH3 concentrations as low as 400 ppb. The sensors showed a very weak response to CO2, CH4, H2, and CO, confirming the selectivity of the hybrids toward ammonia. Hybrids based on rGO and TBPOMPc (M = Cu, Ni, and Pb) were also explored for targeting ammonia.22 The synthesis was carried out through DMF dispersions of MPc and rGO, followed by hydrazine reduction. XPS confirmed the noncovalent functionalization of rGO. Gas sensing performance was tested in the presence of NH3 (0.3–3200 ppm) at room temperature. The resistance typically increased with increasing concentration of NH3. The TBPOMPc/rGO sensors recovered to its original resistance, in contrast to rGO sensors. It was found that TBPOMPc/rGO hybrid sensors were selective toward ammonia when compared to CO, NO2, and H2.
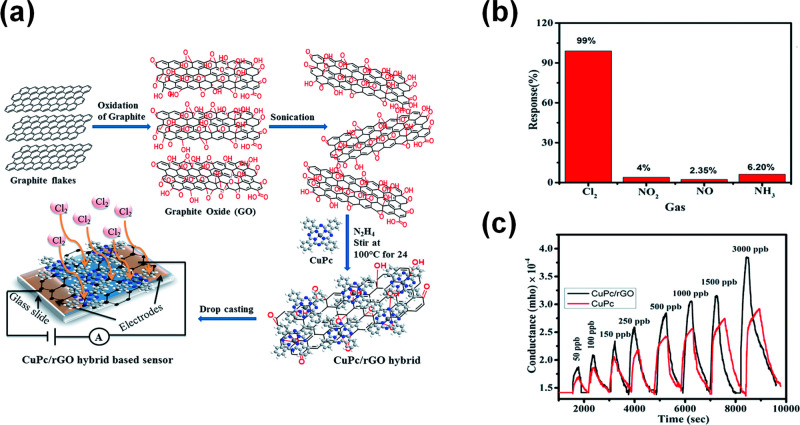
A selective room temperature chemiresistor with a ppb limit of detection for Cl2 was fabricated through the functionalization of rGO with CuPc nanoflowers (Figure 6a).23 Functionalization of rGO was carried out by mixing CuPc powder and GO in DMF, followed by hydrazine reduction. Structural and spectroscopic studies revealed that CuPc islands grow uniformly in a network of nanoflowers lying parallel to the rGO surface. FTIR measurements indicated the delocalization of electrons to form noncovalent interactions between CuPc and the rGO surface by π–π interactions. As illustrated in Figure 6b, the hybrid material presented high selectivity toward Cl2 in comparison to other gases like NO2, NO, and NH3. At low concentrations of 3000 ppb of Cl2, they report a large response characterized by response/recovery time of 190 s/545 s. The sensing mechanism was studied with Raman and electrochemical impedance spectroscopy (EIS), concluding that molecules of Cl2 are physio-adsorbed on the central metal of CuPc. It was noted that the high surface area of the CuPc nanoflowers provides many active sites for Cl2 adsorption, while the rGO provides a conductive network that enhances the sensing performance.
Figure 6.
(a) Schematic representation of synthesized rGO sheets decorated with CuPc nanoflowers. (b) Response histogram of CuPc/rGO hybrid sensor to 500 ppb. (c) Conductance–time plots for different doses of Cl2. Adapted with permission from ref (23). Copyright 2017, Royal Society of Chemistry.
IV. Hybrids Based on Reduced Graphene Oxide/Polypyrrole Molecules
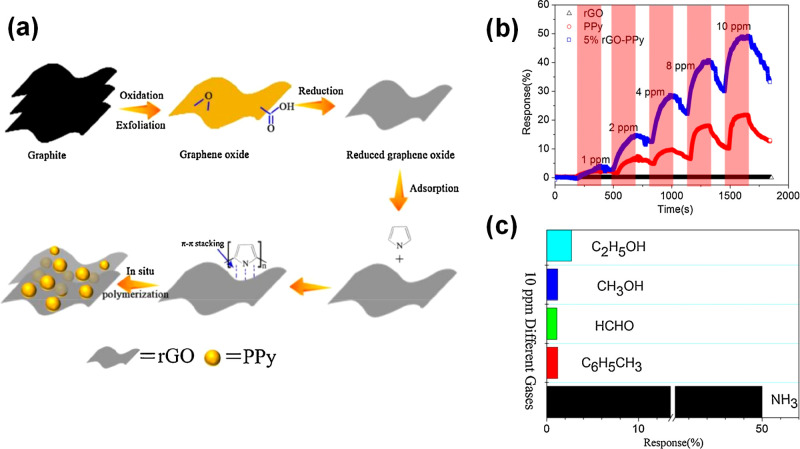
PPy/rGO hybrids were used to develop gas sensors,24 where PPy was attached to rGO through chemical oxidative polymerization (Figure 7a). FTIR and Raman spectroscopy confirmed the π–π interactions of PPy and rGO sheets. Devices based on this hybrid material were evaluated as sensors for ammonia at room temperature, in different concentrations (1 to 10 ppm), showing linear dependence of the response to NH3 concentration (Figure 7b). The sensor selectivity was tested in the presence of VOCs such as toluene, formaldehyde, ethanol, and methanol. The hybrid-based graphene sensor demonstrated relatively low responses to the interfering gases and high response toward ammonia. The proposed sensing mechanism relies on the electron transfer from ammonia to PPy. In addition, due to the H-bonding and π–π stacking interactions in the PPy/rGO hybrid, electron transfer processes can occur between them, leading to increased sensor resistance.
Figure 7.
(a) Mechanism of in situ chemical oxidation polymerization mechanism of PPy on rGO. (b) Response rGO, PPy, and rGO/PPy hybrids to 1–10 ppm of NH3. (c) Selectivity of rGO/PPy to 10 ppm different gases. Adapted with permission from ref (24). Copyright 2017, Elsevier.
In a different approach, rGO/PPy hybrids were prepared by in situ oxidative polymerization.25 Characterization with FTIR and XRD suggested the noncovalent interaction between PPy and rGO by π–π stacking. The gas sensing performance of the hybrid was evaluated at different ammonia concentrations (5–300 ppm) and a general improvement of sensitivity was observed for the PPy/rGO sensors. The high response for ammonia was due to large surface area and porous structure of the composite, which enhances the adsorption of the analyte. PPy and graphene are p-type materials, while NH3 acts as an n-type dopant causing the change in sensor resistance.
Recently, PPy/rGO ammonia sensors were obtained through electrochemical polymerization with a controllable thickness.26 GO was deposited on interdigitated electrodes, followed by thermal reduction; subsequently, a thin layer of PPy was electropolymerized on the rGO surface. The sensing response was evaluated in NH3 concentrations ranging from 1 to 4 ppm and presented a linear response. It was found that the rGO sensors showed noticeably lower response at room temperature in comparison with functionalized hybrid sensors. The recovery time was improved through thermal annealing at 100 °C. The selectivity was also evaluated by exposing the sensor to different analytes such as formaldehyde and H2. The low response to the interferential gases indicated good selectivity toward NH3. The sensing mechanism was attributed to electron transfer between the NH3 molecules and the PPy/rGO hybrid material. Thus, synergistic effects between PPy and rGO were shown to result in significantly higher sensitivity and selectivity than the bare rGO sensor.
V. Hybrids Based on Reduced Graphene Oxide/Donor-π-Acceptor (D-π-A) Molecules
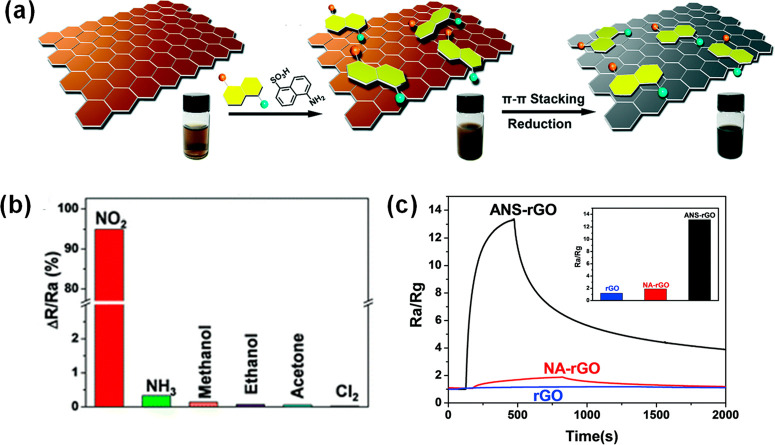
Hybrids based on rGO and a D-π-A molecule ANS–5-aminonaphthalene-1-sulfonic acid were recently reported as a promising sensor for detecting low concentration of NO2.27 The functionalization was carried out by aqueous dispersions of GO and ANS, followed by hydrazine reduction, schematically shown in Figure 8a; the ANS/rGO dispersions were then deposited on electrodes. Raman, FTIR, and XPS confirmed the graphene functionalization by π–π stacking interactions. The sensing performance was evaluated at different NO2 concentrations (1 ppm to 20 ppm), showing linear correlation between the response and NO2 concentration. In addition, the sensor demonstrated selectivity toward NO2, while showing low response for other gases such as nitrogen dioxide, ammonia, methanol, ethanol, acetone, and chlorine as presented in Figure 8b. DFT calculations found that the donor-π-acceptor nature of the ANS molecule facilitates the electron migration, controlling the electrical conductivity of graphene. The extra electrons present on the NH3 group cause the activation of π-electrons on the graphene surface and enhance the charge transfer from graphene to the ANS molecule in the presence of NO2. Thus, the synergetic effect of the ANS/rGO hybrid leads to a high response to NO2. In a similar study, gas sensors based on rGO functionalized with D-π-A molecules: 4HQ–4-hydroxyquinoline and 4AQ–4-aminoquinoline were assessed.28 The material preparation started by mixing GO water dispersions with D-π-A molecules; then, hydrazine was added for obtaining the rGO hybrids. XPS characterization of the hybrids confirmed the presence of the N 1s peaks of the D-π-A molecules suggesting that the molecules were assembled on top of graphene. The sensor response was evaluated for 10 ppm of NO2, and the functionalized rGO sensors were found to have a greater response in comparison with the bare rGO sensor. The selectivity in the presence of interferential gases (NH3, acetone, and Cl2) established that a higher selectivity is achieved for NO2 when using the 4AQ/rGO sensor. DFT calculations assisted in determining the sensing mechanism, which was ascribed to the enhancement of charge transfer due to the high dipole moment of 4AQ-rGO hybrids. NO2 adsorption facilitates the transfer of graphene’s π electrons to the dipolar molecules, enhancing the conductivity of the sensing material.
Figure 8.
(a) Schematic representation of the ANS/rGO hybrid preparation for NO2 gas-sensing applications. (b) Response of ANS/rGO sensing material to 10 ppm of NO2, NH3, methanol, ethanol, acetone, and Cl2. (c) Gas-sensing responses of rGO (1.2), naphthalene-1-sulfonic acid sodium (NA)/rGO (1.9), and ANS-rGO (13.2) upon exposure to 10 ppm of NO2 gas at room temperature. Adapted with permission from ref (27). Copyright 2017, Royal Society of Chemistry.
In Table 1, we summarize the gas sensing performance of the graphene-based hybrids. We focus specifically on the selectivity results from the literature for functionalized gas sensors, and we present a summary in Table 2.
Table 1. Summary of Graphene-Based Hybrids for Gas Sensors and Their Performance Characteristics.
| type of graphene | molecules | analyte gas | concentration (ppm) | response (sensitivity) (%) | recovery time (s) | ref |
|---|---|---|---|---|---|---|
| rGO | 3-CuPc | NH3 | 3200 | 15.4 | - | (18) |
| rGO | cpoPcCo | NH3 | 100 | 42.4 | 450 | (19) |
| rGO | cmpoPcCo | NH3 | 100 | 31.4 | 540 | (19) |
| rGO | poPcCo | NH3 | 100 | 27.3 | 420 | (19) |
| rGO | mpoPcCo | NH3 | 100 | 22.1 | 480 | (19) |
| rGO | ABOPcCo | NH3 | 50 | 23.3 | 250 | (20) |
| rGO | APcCo | NH3 | 50 | 6.4 | 80 | (20) |
| rGO | CuPc | NH3 | 50 | 7.8 | 480 | (21) |
| rGO | NiPc | NH3 | 50 | 5.5 | 300 | (21) |
| rGO | PbPc | NH3 | 50 | 7.8 | 650 | (21) |
| rGO | TBPOCuPc | NH3 | 50 | 8.9 | - | (22) |
| rGO | TBPONiPc | NH3 | 50 | 8.9 | - | (22) |
| CVD | PPy | NH3 | 0.1 ppb | 0.9 | 10 | (16) |
| CVD | PPy | NH3 | 1 | 1.7 | 300 | (17) |
| rGO | ANS | NO2 | 10 | 95.0 | - | (27) |
| rGO | 4AQ | NO2 | 10 | 74.3 | - | (28) |
| rGO | CuPc | Cl2 | 500 ppb | 99 | - | (23) |
| CVD | CoPP | Toluene | 10 | 8.3 | - | (13) |
Table 2. Summary of Graphene-Based Hybrids and Their Selectivity.
| type of graphene | molecules | tested gases | observed selectivity | ref |
|---|---|---|---|---|
| rGO | 3-CuPc | CO2, H2, CH4, CO | NH3 | (18) |
| rGO | cpoPcCo | CO2, H2, CH4, CO, N2, VOCs | NH3 | (19) |
| rGO | cmpoPcCo | CO2, H2, CH4, CO, N2, VOCs | NH3 | (19) |
| rGO | poPcCo | CO2, H2, CH4, CO, N2, VOCs | NH3 | (19) |
| rGO | mpoPcCo | CO2, H2, CH4, CO, N2, VOCs | NH3 | (19) |
| rGO | ABOPcCo | Methanol, ethanol, acetone; dichloromethane; trichloromethane; carbon tetrachloride; toluene; tetrahydrofuran; propionic acid; diethyl ether, ethyl acetate | NH3 | (20) |
| rGO | APcCo | Methanol, ethanol, acetone; dichloromethane; trichloromethane; carbon tetrachloride; toluene; tetrahydrofuran; propionic acid; diethyl ether, ethyl acetate | NH3 | (20) |
| rGO | CuPc | CO2, H2, CH4, CO | NH3 | (21) |
| rGO | NiPc | CO2, H2, CH4, CO | NH3 | (21) |
| rGO | PbPc | CO2, H2, CH4, CO | NH3 | (21) |
| rGO | TBPOCuPc | NO2, H2, CO | NH3 | (22) |
| rGO | TBPONiPc | NO2, H2, CO | NH3 | (22) |
| CVD | Co(tpfpp)ClO4 | Hexane, ethanol, H2O, chloroform, acetonitrile | NH3 | (11) |
| CVD | CoOEP | H2 | NH3 | |
| CVD | PPy | Acetone, water, benzene, toluene, ethanol, naphthalene, chloroform, methanol | NH3, NO2 | (16) |
| CVD | PPy | Formaldehyde, NO2 | NH3 | (17) |
| rGO | PPy | Toluene, formaldehyde, methanol, ethanol | NH3 | (24) |
| rGO | PPy | Formaldehyde, H2 | NH3, NO2 | (26) |
| rGO | ANS | NH3, methanol, ethanol, acetone, Cl2 | NO2 | (27) |
| rGO | 4AQ | Acetone, NH3, Cl2 | NO2 | (28) |
| rGO | CuPc | NO, NH3 | NO2 | (23) |
Conclusions and Outlook
In this mini-review, we surveyed the current progress in the development of selective graphene-based gas sensors by surface functionalization with aromatic molecules and polymers. The functionalized graphene sensors exhibit improved performance compared with those without functionalization in terms of selectivity, sensitivity, and limit of detection. Promising advances in increased selectivity have been achieved for some gases including NH3, NO2, and a variety of VOCs. Functionalization also leads to increased sensitivity, lowering the limit of detection and furthering the efforts for developing commercial technologies. Ammonia is so far the most studied analyte. From the surveyed literature, the cpoCoPc/rGO hybrid presented the lowest detection limit, down to 3.7 ppb of ammonia. On the other hand, at 50 ppm of ammonia, the hybrid ABOPcCo/rGO exhibited the higher response of 23.3%. While progress has been made in the field of functionalized graphene sensors, there remain open questions and challenges.
On the material preparation side, sensor to sensor variation remains a challenge. Standardized methods of graphene transfer and surface cleaning before functionalization are still lacking. Most studies do not present characterization studies of the graphene surface prior to the functionalization, which could be an important determining factor for the device performance. Once materials are prepared, typically spectroscopic techniques are used to infer the molecule–graphene interactions. More effort is needed to fully understand to what extent the surface functionalization is heterogeneous and what are the effects of the heterogeneity. Exploring different methods and parameters for molecular deposition, assisted by scanning probes, could provide such insights. More efforts are also needed for understanding the sensing mechanisms. Theoretical modeling will be invaluable in deciphering the complex charge transfer processes between analyte, functional molecules, and the graphene film. The ability to engineer selective hybrids by judiciously choosing grafted chemical species that target specific analytes will require a deeper understanding of the sensing mechanisms and the specific details of the interaction between the graphene hybrid and the gas analyte. The rapidly evolving field of selective graphene gas sensors will likely focus in the future on detecting other gases beyond the ones explored so far.
In that direction, the difficulty will be to develop methods for increasing the sensitivity toward less interacting analytes. Moreover, the response time of graphene hybrid sensors is less studied than sensitivity or selectivity, yet it can be a critical figure of merit in many applications. More work in understanding the fundamental limits of the response time as well as accurately measuring the response time in graphene sensors will be needed in the future.
One of the main challenges in validating the selectivity of graphene sensors is testing them in realistic conditions under the presence of gas mixtures. For example, environmental sensors should be able to detect a specific target gas in the presence of gases commonly found in air (CO2, H2, or humidity). In applications like breath analysis, biosensors should discern the target analyte from gases like CO2 and VOCs usually present in human breath. In future experiments, it will be important to design sensor testing schemes that provide informative data for selectivity under realistic conditions.
As the field of selective functionalized graphene sensors will evolve, we also anticipate that more efforts will be extended toward the design and implementation of device arrays patterned to detect several molecules at the same time. Combined with features that are already demonstrated such as mechanical flexibility, selectivity, and sensitivity, these chemical noses have the potential to become next-generation environmental monitors.
Acknowledgments
The authors acknowledge support from Department National Defense, Innovation for Defence Excellence and Security (IDEaS) program as well as from NSERC Discovery grant RGPIN-2016-06717.
Glossary
List of Abbreviations
- 3-CuPc
1,8,15,22-tetra-iso-pentyloxyphthalocyanine copper
- Co(tpfpp)ClO4
5,10,15,20-tetrakis(pentafluorophenyl) porphyrinato cobalt(III) perchlorate
- cmpoPcCo
tetra-β-(4-carboxy-3-methoxyphenoxy) phthalocyanine cobalt
- cpoPcCo
tetra-β-carboxylphenoxylphthalocyanine cobalt
- poPcCo
tetra-β-phenoxylphthalocyanine cobalt
- mpoPcCo
tetra-β-(3-methoxyphenoxy) phthalocyanine cobalt
- ABOPcCo
tetra-α-(p-aminobenzyloxy) phthalocyanine cobalt
- APcCo
tetra-α-aminophthalocyanine cobalt
- TBPOMPc
1,8,15,22-tetra-(4-tert-butylphenoxyl)-metallophthalocyanine
- CoOEP
2,3,7,8,12,13,17,18-octaethyl-21H,23H-porphine cobalt(II)
- CoMP
5,10,15,20-meso-tetrakis-nonadecylporphyrin cobalt(II)
- ZnTTPOH
5-(4-hydroxyphenyl)-10,15,20-tri(p-tolyl) zinc porphyrin
- ANS
5-aminonaphthalene-1-sulfonic acid
- 4AQ
4-aminoquinoline
- 4HQ
4-hydroxyquinoline
Biographies
Natalia Alzate-Carvajal received her PhD in Chemistry from the Institute of Applied Science and Technology, National Autonomous University of Mexico in 2017 under the supervision of Prof. Elena Golovataya. She is currently a postdoctoral fellow in the department of Physics at the University of Ottawa, following a postdoctoral fellowship at the University of Montreal. Her research interests are synthesis and characterization of graphene derivatives, functionalization of carbon nanomaterials, and their applications in sensing.
Adina Luican-Mayer is an assistant professor in the Physics Department at the University of Ottawa. She received her PhD in Physics from Rutgers University in 2012, following which she was the Alexei Abrikosov postdoctoral fellow at the Center for Nanoscale Materials at Argonne National Laboratory. Her research group focuses on uncovering the novel electronic properties of low-dimensional systems and devices using scanning probe microscopy and supporting spectroscopic techniques.
The authors declare no competing financial interest.
References
- Hideo A.; Mildred S. D.. Physics of Graphene, 1 ed.; Springer International Publishing: 2014. [Google Scholar]
- Gupta Chatterjee S.; Chatterjee S.; Ray A. K.; Chakraborty A. K. Graphene–metal oxide nanohybrids for toxic gas sensor: A review. Sens. Actuators, B 2015, 221, 1170–1181. 10.1016/j.snb.2015.07.070. [DOI] [Google Scholar]
- Meng F.-L.; Guo Z.; Huang X.-J. Graphene-based hybrids for chemiresistive gas sensors. TrAC, Trends Anal. Chem. 2015, 68, 37–47. 10.1016/j.trac.2015.02.008. [DOI] [Google Scholar]
- Wang T.; Huang D.; Yang Z.; Xu S.; He G.; Li X.; Hu N.; Yin G.; He D.; Zhang L. A Review on Graphene-Based Gas/Vapor Sensors with Unique Properties and Potential Applications. Nano-Micro Lett. 2016, 8 (2), 95–119. 10.1007/s40820-015-0073-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivananju B. N.; Yu W.; Liu Y.; Zhang Y.; Lin B.; Li S.; Bao Q. The Roadmap of Graphene-Based Optical Biochemical Sensors. Adv. Funct. Mater. 2017, 27 (19), 1603918. 10.1002/adfm.201603918. [DOI] [Google Scholar]
- Tian W.; Liu X.; Yu W. Research Progress of Gas Sensor Based on Graphene and Its Derivatives: A Review. Appl. Sci. 2018, 8 (7), 1118. 10.3390/app8071118. [DOI] [Google Scholar]
- Nag A.; Mitra A.; Mukhopadhyay S. C. Graphene and its sensor-based applications: A review. Sens. Actuators, A 2018, 270, 177–194. 10.1016/j.sna.2017.12.028. [DOI] [Google Scholar]
- Mackin C.; Fasoli A.; Xue M.; Lin Y.; Adebiyi A.; Bozano L.; Palacios T. Chemical sensor systems based on 2D and thin film materials. 2D Mater. 2020, 7 (2), 022002. 10.1088/2053-1583/ab6e88. [DOI] [Google Scholar]
- Tarcan R.; Todor-Boer O.; Petrovai I.; Leordean C.; Astilean S.; Botiz I. Reduced graphene oxide today. J. Mater. Chem. C 2020, 8 (4), 1198–1224. 10.1039/C9TC04916A. [DOI] [Google Scholar]
- Georgakilas V.; Otyepka M.; Bourlinos A. B.; Chandra V.; Kim N.; Kemp K. C.; Hobza P.; Zboril R.; Kim K. S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112 (11), 6156–6214. 10.1021/cr3000412. [DOI] [PubMed] [Google Scholar]
- Mackin C.; Schroeder V.; Zurutuza A.; Su C.; Kong J.; Swager T. M.; Palacios T. Chemiresistive Graphene Sensors for Ammonia Detection. ACS Appl. Mater. Interfaces 2018, 10 (18), 16169–16176. 10.1021/acsami.8b00853. [DOI] [PubMed] [Google Scholar]
- Sawada K.; Tanaka T.; Yokoyama T.; Yamachi R.; Oka Y.; Chiba Y.; Masai H.; Terao J.; Uchida K. Co-porphyrin functionalized CVD graphene ammonia sensor with high selectivity to disturbing gases: hydrogen and humidity. Jpn. J. Appl. Phys. 2020, 59 (SG), SGGG09. 10.35848/1347-4065/ab6b80. [DOI] [Google Scholar]
- Pyo S.; Choi J.; Kim J. Improved photo- and chemical-responses of graphene via porphyrin-functionalization for flexible, transparent, and sensitive sensors. Nanotechnology 2019, 30 (21), 215501. 10.1088/1361-6528/ab048d. [DOI] [PubMed] [Google Scholar]
- Iezhokin I.; den Boer D.; Offermans P.; Ridene M.; Elemans J. A. A. W.; Adriaans G. P.; Flipse C. F. J. Porphyrin molecules boost the sensitivity of epitaxial graphene for NH3detection. J. Phys.: Condens. Matter 2017, 29 (6), 065001. 10.1088/1361-648X/29/6/065001. [DOI] [PubMed] [Google Scholar]
- Gajarushi A. S.; Surya S. G.; Walawalkar M. G.; Ravikanth M.; Rao V. R.; Subramaniam C. Ultra-sensitive gas phase detection of 2,4,6-trinitrotoluene by non-covalently functionalized graphene field effect transistors. Analyst 2020, 145 (3), 917–928. 10.1039/C9AN01962F. [DOI] [PubMed] [Google Scholar]
- Yoon T.; Jun J.; Kim D. Y.; Pourasad S.; Shin T. J.; Yu S. U.; Na W.; Jang J.; Kim K. S. An ultra-sensitive, flexible and transparent gas detection film based on well-ordered flat polypyrrole on single-layered graphene. J. Mater. Chem. A 2018, 6 (5), 2257–2263. 10.1039/C7TA10019A. [DOI] [Google Scholar]
- Tang X.; Lahem D.; Raskin J.; Gérard P.; Geng X.; André N.; Debliquy M. A Fast and Room-Temperature Operation Ammonia Sensor Based on Compound of Graphene With Polypyrrole. IEEE Sens. J. 2018, 18 (22), 9088–9096. 10.1109/JSEN.2018.2869203. [DOI] [Google Scholar]
- Zhou X.; Wang X.; Wang B.; Chen Z.; He C.; Wu Y. Preparation, characterization and NH3-sensing properties of reduced graphene oxide/copper phthalocyanine hybrid material. Sens. Actuators, B 2014, 193, 340–348. 10.1016/j.snb.2013.11.090. [DOI] [Google Scholar]
- Guo Z.; Wang B.; Wang X.; Li Y.; Gai S.; Wu Y.; Cheng X. A high-sensitive room temperature gas sensor based on cobalt phthalocyanines and reduced graphene oxide nanohybrids for the ppb-levels of ammonia detection. RSC Adv. 2019, 9 (64), 37518–37525. 10.1039/C9RA08065A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B.; Wang X.; Li X.; Guo Z.; Zhou X.; Wu Y. The effects of amino substituents on the enhanced ammonia sensing performance of PcCo/rGO hybrids. RSC Adv. 2018, 8 (72), 41280–41287. 10.1039/C8RA07509C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Wang B.; Wang X.; Zhou X.; Chen Z.; He C.; Yu Z.; Wu Y. Enhanced NH3-Sensitivity of Reduced Graphene Oxide Modified by Tetra-α-Iso-Pentyloxymetallophthalocyanine Derivatives. Nanoscale Res. Lett. 2015, 10 (1), 373. 10.1186/s11671-015-1072-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z.; Wang B.; Li Y.; Kang D.; Chen Z.; Wu Y. The effect of rigid phenoxyl substituent on the NH3-sensing properties of tetra-α-(4-tert-butylphenoxyl)-metallophthalocyanine/reduced graphene oxide hybrids. RSC Adv. 2017, 7 (36), 22599–22609. 10.1039/C7RA02740K. [DOI] [Google Scholar]
- Kumar S.; Kaur N.; Sharma A. K.; Mahajan A.; Bedi R. K. Improved Cl2 sensing characteristics of reduced graphene oxide when decorated with copper phthalocyanine nanoflowers. RSC Adv. 2017, 7 (41), 25229–25236. 10.1039/C7RA02212C. [DOI] [Google Scholar]
- Sun J.; Shu X.; Tian Y.; Tong Z.; Bai S.; Luo R.; Li D.; Liu C. C. Facile preparation of polypyrrole-reduced graphene oxide hybrid for enhancing NH3 sensing at room temperature. Sens. Actuators, B 2017, 241, 658–664. 10.1016/j.snb.2016.10.047. [DOI] [Google Scholar]
- Tiwari D. C.; Atri P.; Sharma R. Sensitive detection of ammonia by reduced graphene oxide/polypyrrole nanocomposites. Synth. Met. 2015, 203, 228–234. 10.1016/j.synthmet.2015.02.026. [DOI] [Google Scholar]
- Tang X.; Raskin J.-P.; Kryvutsa N.; Hermans S.; Slobodian O.; Nazarov A. N.; Debliquy M. An ammonia sensor composed of polypyrrole synthesized on reduced graphene oxide by electropolymerization. Sens. Actuators, B 2020, 305, 127423. 10.1016/j.snb.2019.127423. [DOI] [Google Scholar]
- Pei W.; Zhang T.; Wang Y.; Chen Z.; Umar A.; Li H.; Guo W. Enhancement of charge transfer between graphene and donor−π-acceptor molecule for ultrahigh sensing performance. Nanoscale 2017, 9 (42), 16273–16280. 10.1039/C7NR04209D. [DOI] [PubMed] [Google Scholar]
- Jia R.; Xie P.; Feng Y.; Chen Z.; Umar A.; Wang Y. Dipole-modified graphene with ultrahigh gas sensibility. Appl. Surf. Sci. 2018, 440, 409–414. 10.1016/j.apsusc.2018.01.166. [DOI] [Google Scholar]