Figure 9.
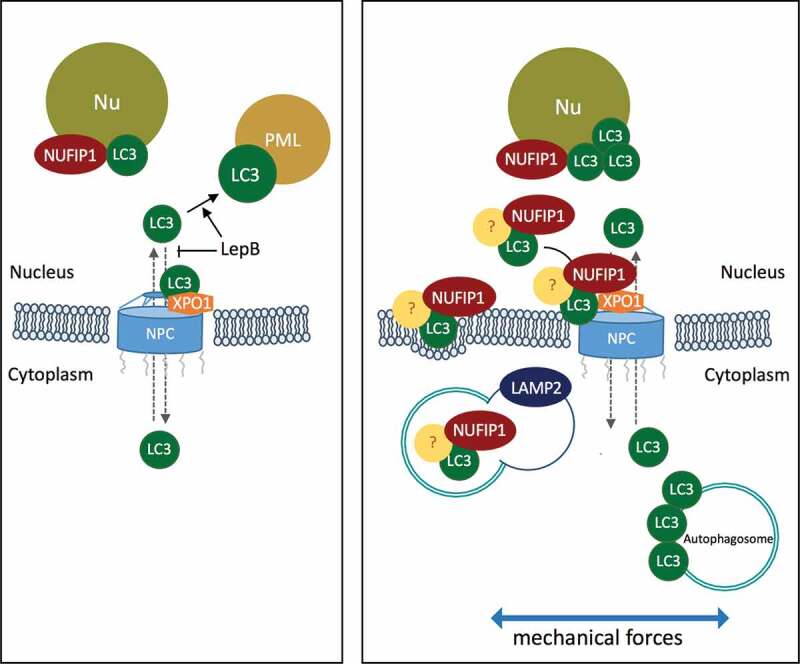
Schematic diagram summarizing the results and working hypothesis. Under non-stretched control conditions, LC3 resides primarily in the cytosol, shuttling in-and-out of the nucleus. LC3 enters the nucleus by passive diffusion and exits in an XPO1-dependent manner. Blockage of active nuclear export by LepB leads to the accumulation of LC3 in the nucleus interacting with PML bodies. Mechanical stress triggers activation of autophagy and nuclear translocation of LC3 to the nucleolus, where it interacts with the autophagy receptor NUFIP1. We propose LC3-NUFIP1 complex to act as a surveillance mechanism that recognizes stretch-induced damaged nuclear proteins and facilitate their export, either via active nuclear transport or nuclear envelope budding, for autophagic degradation in the cytosol.
