Figure 3.
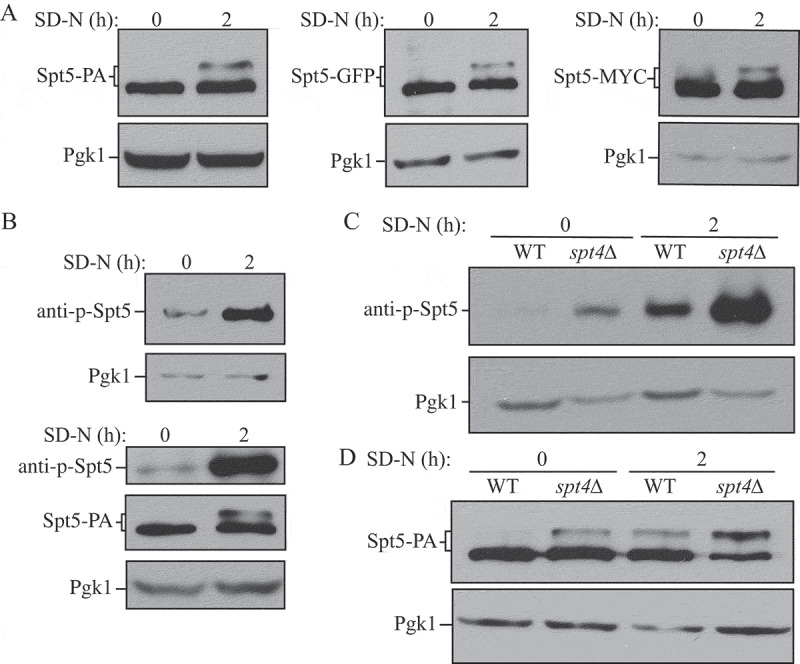
Spt5 phosphorylation is enhanced after starvation or in the absence of Spt4. (A) The protein A (PA)-, GFP- and MYC-tagged Spt5 strains (WXY102, WXY107, DGY047) were collected in growing (YPD, mid-log phase) and starvation (SD-N, t = 2 h) conditions. The samples were separated by SDS-PAGE and detected by western blot with anti-PA, anti-YFP or anti-MYC antibodies. Anti-Pgk1 antiserum was used to detect the loading control. (B) Wild-type (SEY6210) cells were grown to mid-log phase in growing conditions (YPD) and then starved (SD-N) for 2 h. Samples under these 2 conditions were collected and analyzed by western blot. An antibody specific for the phosphorylated form of Spt5 (anti-p-Spt5) was used to detect the phosphorylated band (upper blot). We also examined phosphorylation in a strain expressing Spt5 tagged with PA (WXY102); the total protein level of Spt5 was detected with anti-PA and the phosphorylation of Spt5 was monitored with the anti-p-Spt5 antibody (lower blot) (C) Samples from wild-type (WLY176) and spt4∆ (WXY105) strains were collected in both growing (YPD) and starvation (SD-N, t = 2 h) conditions for western blot analysis. Spt5 phosphorylation was monitored with the anti-p-Spt5 antibody. (D) The Spt5-PA level of wild-type (WXY102) and spt4∆ (WXY103) cells in growing (YPD) and starvation (SD-N) conditions was detected by western blot with anti-PA antibodies.
