Figure 4.
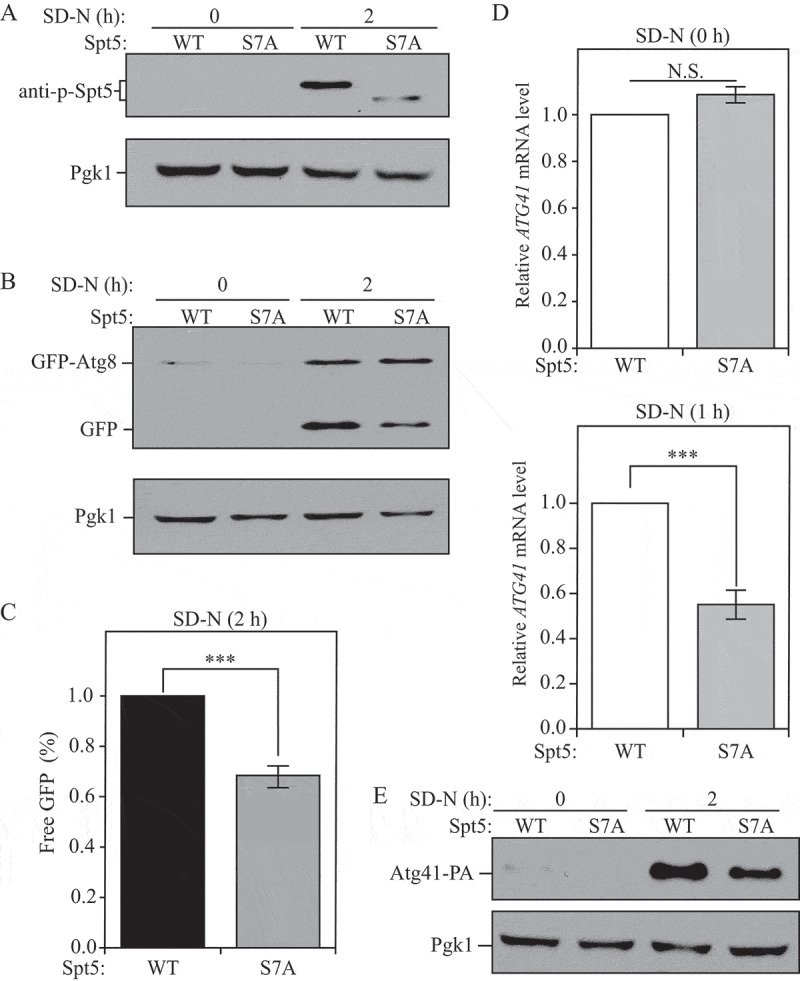
Dephosphoryaltion of Spt5 negatively modulates autophagy by downregulating ATG41 mRNA and protein levels after starvation. (A) Samples collected from cells expressing WT (DGY047) and nonphosphorylable Spt5 (DGY048) were analyzed by western blot. The phosphorylation of Spt5 was detected with the specific anti-p-Spt5 antibody. (B-C) Autophagy activity was measured by GFP-Atg8 processing in WT (DGY050) and Spt5[S7A] (DGY051) cells harboring endogenous GFP-ATG8 plasmids under growing conditions and after 2 h of starvation. The quantitative analysis of processed GFP after starvation (there is no processed GFP in the growing condition) is shown in (C), and the error bar represents the SEM of 3 independent experiments. The processed GFP of the WT strain after starvation was set as 1, and other values were normalized. ***, p < 0.005. (D) The mRNA level of ATG41 in WT (DGY047) and Spt5[S7A] (DGY048) cells was measured by RT-qPCR under growing conditions and starvation (SD-N, 1 h). The error bar represents the SEM of 3 independent experiments, and p values are reported for the comparison between the wild-type and Spt5[S7A] strains during starvation. N.S., not significant, ***, p < 0.005. (E) The anti-PA antibody was used to detect Atg41-PA in samples (WT, WXY127; Spt5[S7A], WXY128) collected from growing and starvation (SD-N, 2 h) conditions by western blot analysis.
