Figure 1.
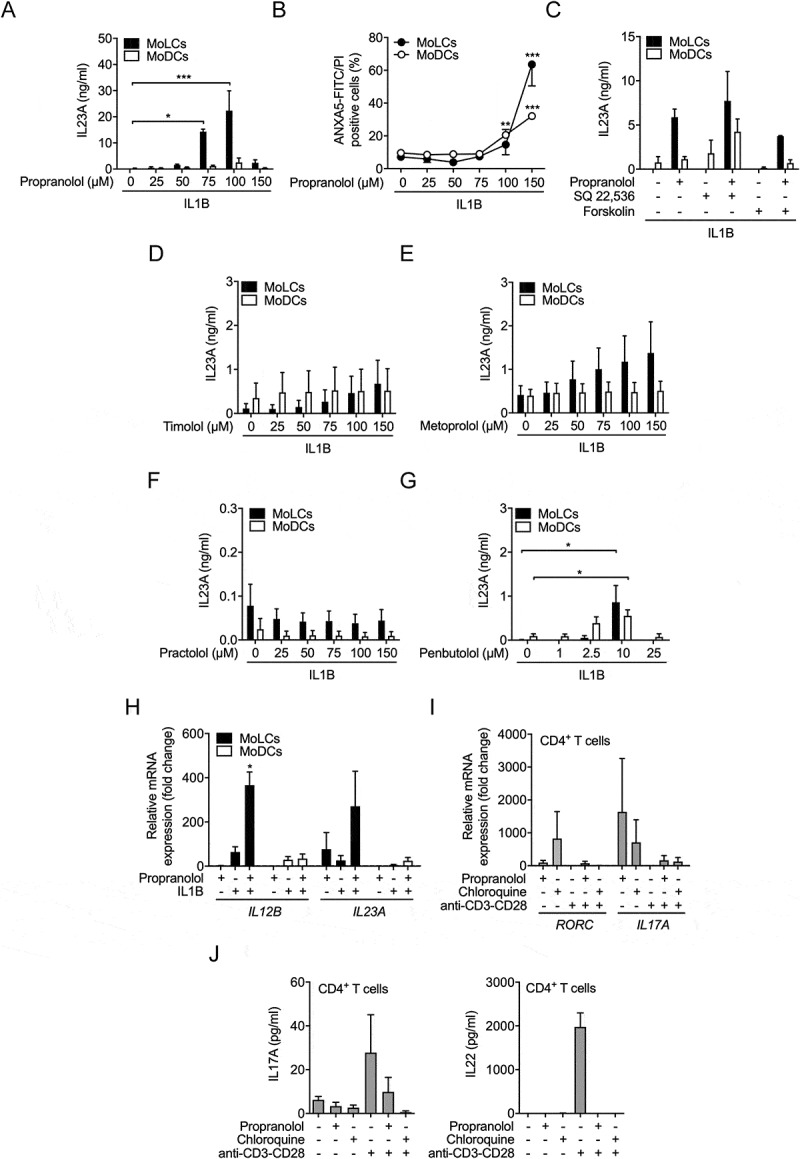
Lysosomotropic beta-blocker induce IL23A release by activated MoLCs independent of beta adrenoceptors. (A) IL23A secretion was analyzed by ELISA in the supernatant of MoLCs and MoDCs stimulated with IL1B (20 ng/ml) for 24 h in the presence or absence of propranolol at different concentrations (25–150 µM). (B) Cell viability was assessed by ANXA5-FITC/PI-double-staining using flow cytometry. (C) IL23A release by MoLCs and MoDCs, quantified after stimulation with SQ 22,536 (100 µM) or forskolin (20 µM) and followed by activation with IL1B (20 ng/ml) for 24 h in the presence or absence of propranolol (75 µM). (D-G) IL23A production by MoLCs and MoDCs after stimulation with IL1B (20 ng/ml) for 24 h in the presence or absence of timolol, metoprolol, practolol or penbutolol at different concentrations (1–150 µM) was assayed by ELISA. (H) mRNA expression of IL12B and IL23A in MoLCs and MoDCs, respectively, was assessed after 24 h of stimulation with IL1B (20 ng/ml) in the presence or absence of propranolol (75 µM). Gene expression results were normalized to GAPDH and depicted relative to unstimulated MoLCs and MoDCs (set as 1.0). Propranolol does not directly polarize naïve CD4+ T cells toward Th17 development. (I) Gene expression levels of RORC and IL17A were examined in naïve CD4+ T cells, polyclonally stimulated with anti-CD3-CD28 antibodies for 5 d with or without propranolol (75 µM) or chloroquine (20 µM). Levels of mRNA were normalized to GAPDH and presented relative to untreated CD4+ T cells (set as 1.0). (J) Naïve CD4+ T cells were stimulated with anti-CD3-CD28 antibodies for 7 d in presence or absence of propranolol (75 µM) or chloroquine (20 µM), respectively. Th17 signature cytokines IL17A and IL22 in cell culture supernatants were quantified by ELISA. (A-G) *P < 0.05, **P < 0.01, ***P < 0.001, one-way ANOVA test followed by Bonferroni posttest, (H and I) one-sample t-test. Data are representative of (A) n = 4–5, (B) n = 3–6, (C) n = 3–5, (D-J) n = 3 independent experiments and display mean values ± SEM.
