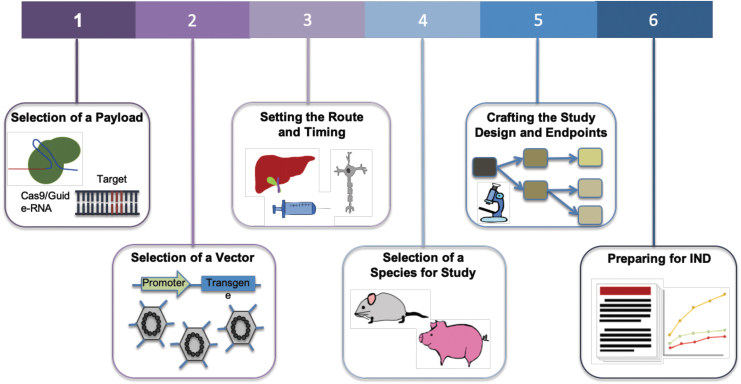
FIG. 1.
Steps toward enabling a gene therapy via CRISPR-Cas. For a disease target, the choice of Cas effector and corresponding gRNA is the first step. Next is to determine CRISPR-Cas delivery options via viral or nonviral methods. Associated with delivery system is also the route of administration. This typically depends on the organ or tissue of treatment and ideally should be limited to that location. Studies are then performed to assess the distribution, persistence, and clearance of the vector and the expression of the therapy in target tissues. Relevant clinical insight is also dependent on the selection of species for study, and a preclinical study design is crafted. Clinical endpoints are set based on safety and toxicology studies. Proof of safety and efficacy in preclinical studies will then lead to an IND application. gRNA, guide-RNA; IND, Investigational New Drug.