Abstract
Tirabrutinib is a second‐generation Bruton’s tyrosine kinase inhibitor with greater selectivity than ibrutinib. Here, we conducted a multicenter, phase II study of tirabrutinib in patients with treatment‐naïve (Cohort A) or with relapsed/refractory (Cohort B) Waldenström’s macroglobulinemia (WM). Patients were treated with tirabrutinib 480 mg once daily. The primary endpoint was major response rate (MRR; ≥ partial response). Secondary endpoints included overall response rate (ORR; ≥ minor response), time to major response (TTMR), progression‐free survival (PFS), overall survival (OS), and safety. In total, 27 patients (18 in Cohort A; 9 in Cohort B) were enrolled. The median age was 71 y, and the median serum immunoglobulin M level was 3600 mg/dL. Among the patients, 96.2% had the MYD88L265P mutation. MRR and ORR were 88.9% and 96.3%, respectively (Cohort A: MRR, 88.9%; ORR, 94.4%; Cohort B: MRR, 88.9%; ORR, 100%). Median TTMR was 1.87 mo. PFS and OS were not reached with a median follow‐up of 6.5 and 8.3 mo for Cohorts A and B, respectively. The most common adverse events (AEs) were rash (44.4%), neutropenia (25.9%), and leukopenia (22.2%), with most AEs classified as grade 1 or 2. Grade ≥ 3 AEs included neutropenia (11.1%), lymphopenia (11.1%), and leukopenia (7.4%). No grade 5 AEs were noted. All bleeding events were grade 1; none were associated with drug‐related atrial fibrillation or hypertension. Although the follow‐up duration was relatively short, the study met the primary endpoint. Therefore, tirabrutinib monotherapy is considered to be highly effective for both untreated and relapsed/refractory WM with a manageable safety profile. (JapicCTI‐173646).
Keywords: B‐cell malignancy, BTK inhibitor, Japanese, tirabrutinib, Waldenström’s macroglobulinemia
We conducted a multicenter, phase II study of tirabrutinib in patients with treatment‐naïve or with relapsed/refractory Waldenström’s macroglobulinemia. Although the follow‐up duration was relatively short, the study met the primary endpoint. Tirabrutinib monotherapy is considered to be highly effective for both untreated and relapsed/refractory WM with a manageable safety profile.

Abbreviations
- AE
adverse event
- AS‐PCR
allele‐specific polymerase chain reaction
- BTK
Bruton’s tyrosine kinase
- CI
confidential interval
- CLL
chronic lymphocytic leukemia
- CR
complete response
- CT
computed tomography
- ECOG PS
Eastern Cooperative Oncology Group performance status
- EGFR
epidermal growth factor receptor
- IPSSWM
International Prognostic Scoring System for Waldenström’s macroglobulinemia
- IRC
independent review committee
- MR
minor response
- MRR
major response rate
- NGS
next‐generation sequencing
- ORR
overall response rate
- OS
overall survival
- PD
progressive disease
- PFS
progression‐free survival
- PR
partial response
- SPD
sum of the products of the greatest diameters
- TTMR
time to major response
- TTOR
time to overall response
- VGPR
very good partial response
- WHIM
warts, hypogammaglobulinemia infection, and myelokathexis syndrome
- WM
Waldenström’s macroglobulinemia
1. INTRODUCTION
Waldenström’s macroglobulinemia (WM) is a major type of lymphoplasmacytic lymphoma that is characterized by the infiltration of neoplastic B cells with plasmacytic differentiation into bone marrow and IgM monoclonal gammopathy. 1 , 2 Several treatment options for WM have been developed, including rituximab monotherapy, chemoimmunotherapy, and bortezomib‐based treatments. 3 , 4 , 5 , 6 , 7 , 8 However, WM typically progresses despite treatment in most patients. In addition, hematologic and non‐hematologic toxicities, including peripheral neuropathy, infections, and secondary malignancies, remain major concerns for these treatments. 9 Therefore, the development of new treatment strategies is necessary.
BTK is predominantly expressed in B cells, where it plays a critical role in B‐cell development and function. 10 , 11 , 12 , 13 However, in B‐cell lymphoma, the BTK‐mediated signal cascade is constitutively activated, 14 and knockdown of BTK is shown to reduce the survival of malignant B cells. In WM, a mutation in MYD88L265 serves to support the survival of lymphoplasmacytic cells via BTK activation. This mutation (MYD88L265P) has been found to be present in the majority of patients with WM 15 , 16 and triggers nuclear factor kappa B activation via phosphorylated BTK. 17 Therefore, BTK inhibition is considered a promising therapeutic option for WM.
Ibrutinib is an irreversible selective inhibitor for BTK and has been shown to be highly effective for WM. 18 , 19 A randomized phase III trial demonstrated a higher response rate in patients treated with ibrutinib and rituximab compared with those treated with rituximab alone (iNNOVATE Study). 20 However, safety remains a major concern regarding ibrutinib treatment, with several adverse effects noted, including atrial fibrillation, bleeding events, and hypertension. 21 , 22 , 23 , 24 , 25 This is likely because ibrutinib has some off‐target effects, including inhibition of other tyrosine kinases such as EGFR, tyrosine kinase expressed in hepatocellular carcinoma, the phosphoinositide 3‐kinase/AKT cardio protective pathway, and interleukin‐2‐inducible T‐cell kinase. 26 A systematic review and meta‐analysis of ibrutinib treatment for patients with CLL demonstrated an increased risk of these toxicities, which led to the discontinuation of ibrutinib. 22 , 23 Thus, the development of an effective treatment for WM with a reduced incidence of these toxicities would contribute to a longer response duration.
Tirabrutinib (ONO/GS‐4059) is a highly potent and selective second‐generation BTK inhibitor. 27 , 28 Tirabrutinib forms a covalent bond with BTK and has demonstrated effective in vitro cytotoxicity in B‐cell lymphoma and in vivo antitumor activity in mouse models. 27 Multicenter, dose‐escalation phase I trials of tirabrutinib for relapsed or refractory CLL and B‐cell lymphoma, including WM, have demonstrated the efficacy and tolerability of tirabrutinib. 29 , 30
Therefore, we conducted a multicenter, open‐label, phase II study of tirabrutinib to evaluate its efficacy and safety in patients with WM.
2. MATERIALS AND METHODS
2.1. Trial design and treatment
This phase II trial (Japan Pharmaceutical Information Center clinical trial ID: JapicCTI‐173646) was conducted with an open‐label and single‐arm design at 19 sites in Japan. The enrollment of patients began on November 8, 2018 and closed on February 22, 2019. All authors had full access to the primary data.
Tirabrutinib was administered orally under fasting conditions at a daily dose of 480 mg for 28 d as 1 cycle. Tirabrutinib was continued until disease progression or clinically unacceptable toxicity. The dose was determined based on safety and efficacy data obtained in previous clinical trials. 29 , 30 During the study, the administration of tirabrutinib would be interrupted due to AEs and, after recovery, a reduced dose of 320 or 160 mg/d would be administered at the physician’s discretion.
2.2. Patients
Patients histologically diagnosed with WM were enrolled into 2 cohorts. Patients in Cohort A were treatment‐naïve patients, and those in Cohort B were relapsed or refractory patients who received 1 or more lines of systemic treatment for WM. The inclusion criteria in Cohort A were either presence of symptomatic WM 31 , 32 or serum IgM levels of >4000 mg/dL. Other major eligibility criteria for both cohorts included age ≥ 20 y, monoclonal gammopathy with serum IgM levels of >500 mg/dL, an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0 or 1, and acceptable laboratory test results. The International Prognostic Scoring System for WM (IPSSWM) score was also categorized. 33 Major exclusion criteria were tumor lesions in the central nervous system and prior administration of BTK inhibitors.
2.3. MYD88 and CXCR4 genotyping
Genomic DNA was isolated from bone marrow and/or tumor tissues. The L265P mutation in MYD88 was specifically assessed by real‐time polymerase chain reaction using an allele‐specific oligonucleotide (AS‐PCR). 34 Mutations in CXCR4 were analyzed using the Ion Torrent PGM NGS system (Life Technologies Japan Ltd.). 35 The occurrence of warts, hypogammaglobulinemia infection, and myelokathexis syndrome (WHIM)‐like mutations in CXCR4, 36 , 37 hereafter referred to as CXCR4WHIM, were a particular focus of this study.
2.4. Efficacy and safety
The primary endpoint of this study was MRR, as assessed by an IRC according to the consensus criteria of the VIth International Workshop for Waldenström’s Macroglobulinemia. 38 The consensus categories for the response comprised CR, VGPR, PR, MR, stable disease, and PD. Major response was defined to include CR, VGPR, and PR. The overall response was defined to include the major response and MR. The major secondary endpoints were the ORR, TTMR, TTOR, duration of major response, PFS, OS, decreases in serum IgM and in the sizes of measurable lesions in the lymph nodes and extramedullary diseases by CT, and changes in primary disease‐associated clinical symptoms, including hemoglobin levels. The lesion size was defined as the sum of the products of the greatest diameters (SPD). Serum IgM and SPD were monitored before the first administration, at the end of the first cycle, and at the beginning of each odd‐numbered cycle.
AEs that occurred during administration, within 28 d after completion of administration of the study drug, and before the start of a subsequent therapy were evaluated, as were death and the data cutoff point. AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. 39
2.5. Statistics
The expected MRR of Cohorts A and B were 80.0% and 70.0%, respectively. The required number of patients in each cohort was 18 in Cohort A and 8 in Cohort B. This was calculated so that the study would show that the lower limit of the 95% CI for the MRR was higher than the threshold response rates of 45.0% (Cohort A) and 20.0% (Cohort B), with a probability of at least 80%. 40 , 41 , 42
Baseline characteristics were analyzed for all enrolled patients, including the efficacy in patients having one or more response scores as evaluated by the IRC after the administration of tirabrutinib and the safety in patients administered with at least 1 dose of tirabrutinib. The data cutoff point was August 28, 2019, which was the first day of the seventh cycle for the latest‐enrolled patient. The Clopper‐Pearson method was used to estimate the 95% CIs for the MRR and ORR. No adjustments were made for covariates. The maximum reduction ratios of serum IgM levels and the measurable lesion sizes by CT were assessed using waterfall plots.
3. RESULTS
3.1. Patient characteristics
Among the 30 patients who provided informed consent, 18 treatment‐naïve patients with WM were enrolled in Cohort A and 9 relapsed or refractory patients were enrolled in Cohort B (Figure 1; Table 1). All 27 patients met the criteria for efficacy and safety analyses. At median follow‐ups of 6.5 and 8.3 mo for Cohort A and Cohort B, respectively, 24 patients continued the administration (Figure 2). One patient in Cohort B discontinued the administration due to PD, 1 in Cohort A discontinued due to an AE, and another in Cohort A discontinued at the physician’s discretion following the patient’s request of study drug discontinuation after PR.
FIGURE 1.
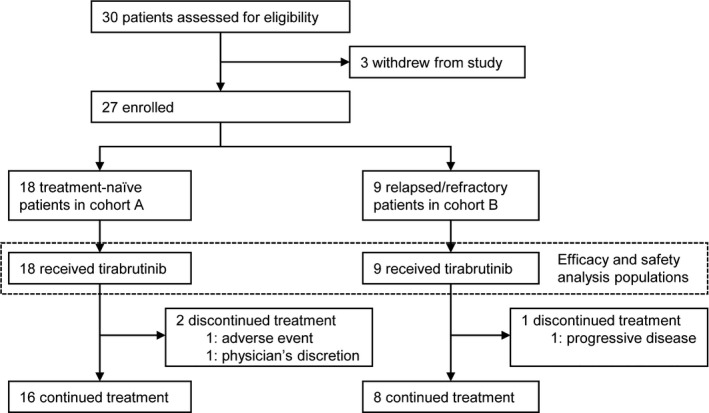
Patient disposition
TABLE 1.
Baseline demographic and disease characteristics
|
Cohort A Treatment‐naïve N = 18 |
Cohort B Relapsed/refractory N = 9 |
Total N = 27 |
|
|---|---|---|---|
| Sex | |||
| Female | 3 (16.7) | 2 (22.2) | 5 (18.5) |
| Male | 15 (83.3) | 7 (77.8) | 22 (81.5) |
| Age | |||
| Median (y) | 70.5 (50‐82) | 71 (60‐83) | 71 (50‐83) |
| IPSSWM | |||
| Low risk | 3 (16.7) | 2 (22.2) | 5 (18.5) |
| Intermediate risk | 8 (44.4) | 5 (55.6) | 13 (48.1) |
| High risk | 7 (38.9) | 2 (22.2) | 9 (33.3) |
| Serum IgM | |||
| Median (mg/dL) | 3787.5 (1392‐6340) | 2105.0 (730‐6930) | 3600.0 (730‐6930) |
| ≤4000 mg/dL | 11 (61.1) | 7 (77.8) | 18 (66.7) |
| >4000 mg/dL | 7 (38.9) | 2 (22.2) | 9 (33.3) |
| Hemoglobin | |||
| Median (g/dL) | 10.45 (8.0‐15.3) | 12.20 (9.1‐13.9) | 10.60 (8.0‐15.3) |
| Platelet count | |||
| Median (109/L) | 257.5 (67‐441) | 206.0 (79‐311) | 257.0 (67‐441) |
| β2‐microglobulin | |||
| Median (mg/L) | 3.150 (1.60‐9.70) | 3.200 (1.70‐5.50) | 3.200 (1.60‐9.70) |
| Lymphadenopathy, Yes | 8 (44.4) | 6 (66.7) | 14 (51.9) |
| Splenomegaly, Yes | 10 (55.6) | 4 (44.4) | 14 (51.9) |
| Hyperviscosity, Yes | 6 (33.3) | 1 (11.1) | 7 (25.9) |
| Gene mutations a | |||
| MYD88L265P | 16 (94.1) | 9 (100.0) | 25 (96.2) |
| CXCR4WHIM | 4 (23.5) | 0 | 4 (15.4) |
| MYD88WT/ CXCR4WHIM | 1 (5.9) | 0 | 1 (3.8) |
| MYD88L265P/ CXCR4WT | 13 (76.5) | 9 (100.0) | 22 (84.6) |
| MYD88L265P/ CXCR4WHIM | 3 (17.6) | 0 | 3 (11.5) |
| Number of prior therapies | |||
| Median (range) | NA | 2.0 (1‐7) | NA |
| 1 | NA | 3 (33.3) | NA |
| 2 | NA | 3 (33.3) | NA |
| ≥3 | NA | 3 (33.3) | NA |
| Prior therapies | |||
| Rituximab | NA | 8 (88.9) | NA |
| Bortezomib | NA | 3 (33.3) | NA |
| Bendamustine | NA | 3 (33.3) | NA |
| Other alkylating agents | NA | 6 (66.7) | NA |
Data are numbers of patients (%) or median (range).
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; IPSSWM, International Prognostic Staging System for Waldenström’s Macroglobulinemia; NA, not applicable.
Gene mutation data were missing in 1 patient in Cohort A.
FIGURE 2.
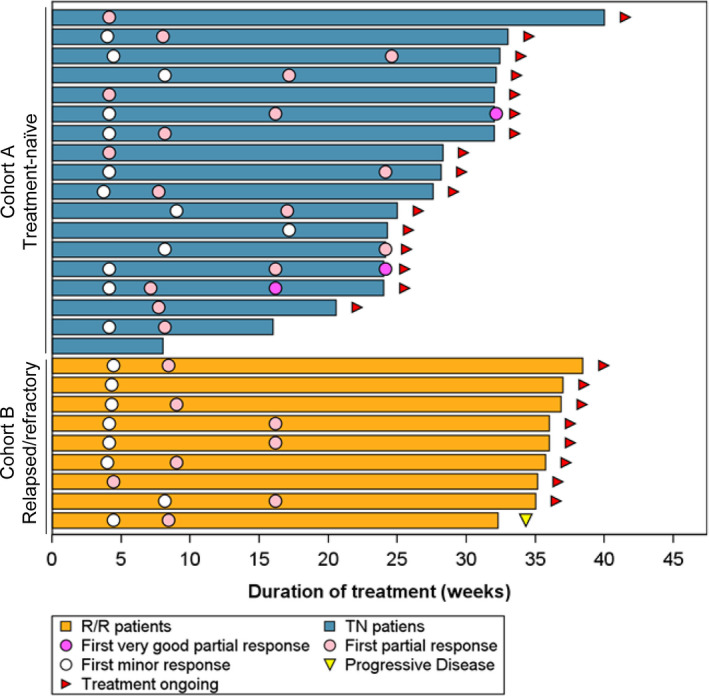
Duration of treatment and responses. A swimmer plot shows the duration of treatment, the first timings of better responses, and a progressive disease analysis for each patient
The baseline demographics, clinical characteristics, baseline laboratory values, IPSSWM scores, and genotypes of the enrolled patients are listed in Table 1. The overall median age was 71 y (range, 50‐83), with the median age of Cohort A 70.5 y (range, 50‐82) and that of Cohort B 71 y (range, 60‐83). The overall median baseline serum IgM level was 3600 mg/dL (range, 730‐6930), with that of Cohort A 3788 mg/dL (range, 1392‐6340) and that of Cohort B 2105 mg/dL (range, 730‐6930). The number of patients in whom the baseline serum IgM level was >4000 mg/dL was 9 (33.3%), with 7 (38.9%) in Cohort A and 2 (22.2%) in Cohort B. Prior systemic therapies administered to Cohort B included rituximab in 8 patients (88.9%), bortezomib in 3 patients (33.3%), bendamustine in 3 patients (33.3%), and other alkylating agents in 6 patients (66.7%). No patients received purine analogs, such as fludarabine, or autologous transplantation. The majority (96.2%) of the patients (16 in Cohort A and 9 in Cohort B) were identified to have a mutation in MYD88.
3.2. Efficacy
The MRR and ORR in all patients were 88.9% and 96.3%, respectively. The MRR was 88.9% in both Cohort A (16 patients, including 3 patients exhibiting VGPR; 95% CI, 65.3‐98.6) and Cohort B (8 patients; 95% CI, 51.8‐99.7) (Table 2). The ORRs were 94.4% (17 patients; 95% CI, 72.7‐99.9) and 100% (9 patients; 95% CI, 66.4‐100.0) in Cohorts A and B, respectively. The median TTOR and TTMR in all patients were 0.95 (range, 0.9‐3.9) and 2.00 (range, 1.0‐5.7) mo; in Cohort A, 0.95 (range, 0.9‐3.9) and 1.87 (range, 1.0‐5.7) mo; and in Cohort B, 0.99 (range, 0.9‐1.9) and 2.07 (range, 1.0‐3.7) mo, respectively (Figure 2; Table 2). Because all the major responders, with 1 exception, achieved a major response by the data cutoff point, the median duration of the major response was not reached in either cohort.
TABLE 2.
IRC‐assessed responses
| Genotype | Cohort A N = 18 | Cohort B N = 9 | ALL N = 27 | ALL N = 26 a | ||
|---|---|---|---|---|---|---|
| All N = 18 | All N = 9 | All N = 27 | MYD88WT CXCR4WHIM N = 1 | MYD88L265P CXCR4WT N = 22 | MYD88L265P CXCR4WHIM N = 3 | |
| Response rates – % (95% CI) | ||||||
| MRR (CR + VGPR + PR) | 88.9 (65.3‐98.6) | 88.9 (51.8‐99.7) | 88.9 (70.8‐97.6) | 100 (2.50‐100) | 90.9 (70.8‐98.9) | 66.7 (9.43‐99.2) |
| ORR (CR + VGPR + PR + MR) | 94.4 (72.7‐99.9) | 100 (66.4‐100) | 96.3 (81.0‐99.9) | 100 (2.50‐100) | 95.5 (77.2‐99.9) | 100 (29.2‐100) |
| Best overall response – n (%) | ||||||
| CR | 0 | 0 | 0 | 0 | 0 | 0 |
| VGPR | 3 (16.7) | 0 | 3 (11.1) | 0 | 3 (13.6) | 0 |
| PR | 13 (72.2) | 8 (88.9) | 21 (77.8) | 1 (100) | 17 (77.3) | 2 (66.7) |
| MR | 1 (5.6) | 1 (11.1) | 2 (7.4) | 0 | 1 (4.5) | 1 (33.3) |
| SD | 1 (5.6) | 0 | 1 (3.7) | 0 | 1 (4.5) | 0 |
| PD | 0 | 0 | 0 | 0 | 0 | 0 |
| Median TTMR – mo (range) | 1.87 (1.0‐5.7) | 2.07 (1.0‐3.7) | 2.00 (1.0‐5.7) | 5.55 (5.6‐5.6) | 1.94 (1.0‐5.6) | 2.89 (1.9‐3.9) |
| Median TTOR — mo (range) | 0.95 (0.9‐3.9) | 0.99 (0.9‐1.9) | 0.95 (0.9‐3.9) | 0.95 (1.0‐1.0) | 0.95 (0.9‐1.9) | 2.07 (1.0‐3.9) |
Abbreviations: 95% CI, 95% confidential interval; CR, complete response; MR, minor response; MRR, major response rate; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease; TTMR, time to major responses; TTOR, time to overall responses; VGPR, very good partial response
Gene mutation data were missing in 1 patient in Cohort A.
The L265P mutation in MYD88, as assessed by AS‐PCR, was found in 94.1% and 100% of patients in Cohorts A and B, respectively, whereas CXCR4WHIM, as assessed by NGS, was only found in 23.5% of patients in Cohort A (Table 1). The IRC‐assessed responses in the patient subpopulations were classified according to the mutations in MYD88 and CXCR4 and are summarized in Table 2 and Supporting Information, Table S1. Briefly, the MRRs in patients with MYD88L265 P/CXCR4WT (90.9%; 95% CI, 70.8‐98.9) were relatively higher than those in patients with MYD88L265P/CXCR4WHIM (66.7%; 95% CI, 9.43‐99.2). The median TTMRs were comparable between patients with MYD88L265P/CXCR4WT (1.94 mo) and those with MYD88L265P/CXCR4WHIM (2.89 mo).
Regarding PFS and OS, no events were observed during the study period, with the exception that 1 patient (11.1%) in Cohort B, whose best overall response was PR, had PD at 240 d after the start of the study drug administration. All patients in both cohorts experienced a decrease in serum IgM levels (Figure 3A). The best reductions in individual serum IgM levels were greater than 50% in 25 (92.6%) patients in total, with 16 (88.9%) and 9 (100%) patients in Cohorts A and B, respectively. Among 14 patients in total with prior lymphadenopathy, SPD data were available for 13 patients (8 and 5 patients in Cohorts A and B, respectively). All the patients experienced marked shrinkage in lesion size, with > 50% best reduction in SPD observed in 87.5% and 80% of patients in Cohorts A and B, respectively (Figures 3B and S1). Low basal levels of hemoglobin, particularly in Cohort A, recovered during the treatment (Figure 3C).
FIGURE 3.
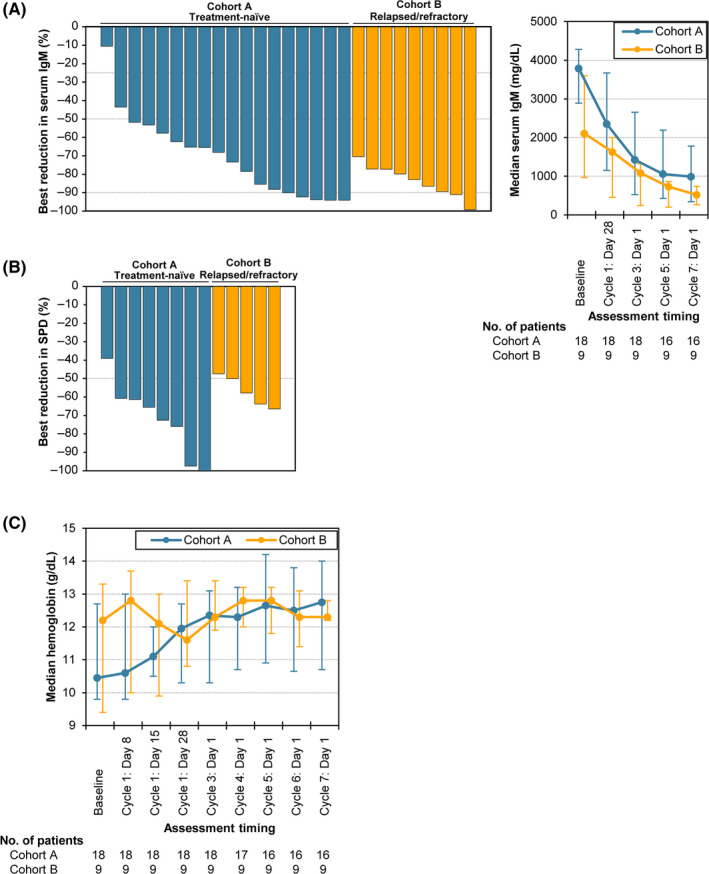
Best reductions in serum IgM and SPD and chronological changes in median hemoglobin levels. A, Left panel: A waterfall plot shows the best reductions in serum IgM level in each patient. Dotted lines represent 25%, 50%, and 90%, which are the thresholds of each response. Right panel: Chronological changes in median serum IgM levels are shown. The error bars represent the interquartile ranges. B, The best reductions in SPD are shown as a waterfall plot. The SPD data for 1 patient in Cohort B were missing. C, Chronological changes in median hemoglobin levels are shown. The error bars represent the interquartile ranges
Baseline clinical symptoms associated with WM were observed in 21 patients in total, with 16 and 5 patients in Cohorts A and B, respectively (Table S2). Among them, 15 patients (71.4%) in total, including 11 (68.8%) in Cohort A and 4 (80.0%) in Cohort B, experienced resolution of all symptoms at least once after administration of the study drug.
3.3. Safety
All patients experienced AEs. The common AEs were rash (44.4%), neutropenia (25.9%), leukopenia (22.2%), and stomatitis (14.8%) (Table 3). Grade 3 and 4 AEs were observed in 8 patients (29.6%), including 3 with neutropenia (11.1%), 3 with lymphopenia (11.1%), and 2 with leukopenia (7.4%). No grade 5 AEs were identified. Serious AEs, including transient ischemic attack on day 49, classified as grade 2, and rhegmatogenous retinal detachment on day 184, classified as grade 3, were observed in 2 patients in Cohort A (7.4%). These serious AEs appeared to be unrelated to tirabrutinib treatment, and the patients recovered after treatment interruption. Three patients experienced tirabrutinib‐related grade 1 bleeding events, including epistaxis (2 patients) and mouth hemorrhage (1 patient). These patients continued the study drug administration without interruption but were administered a reduced dose of tirabrutinib. One patient was considered to have worsening chronic atrial fibrillation; however, electrocardiography and echocardiography revealed no apparent changes. The chronic atrial fibrillation was considered to be due to exercise reported by the patient and not due to drugs.
TABLE 3.
Common adverse events
| Adverse events — n (%) | Cohort A Treatment‐naïve N = 18 | Cohort B Relapsed/refractory N = 9 | Total N = 27 | |
|---|---|---|---|---|
| All | 18 (100) | 9 (100) | 27 (100) | |
| Grade ≥ 3 | 4 (22.2) | 4 (44.4) | 8 (29.6) | |
| Rash | 11 (61.1) | 1 (11.1) | 12 (44.4) | |
| Neutropenia | 2 (11.1) | 5 (55.6) | 7 (25.9) | |
| Grade ≥ 3 | 0 | 3 (33.3) | 3 (11.1) | |
| Leukopenia | 2 (11.1) | 4 (44.4) | 6 (22.2) | |
| Grade ≥ 3 | 0 | 2 (22.2) | 2 (7.4) | |
| Stomatitis | 3 (16.7) | 1 (11.1) | 4 (14.8) | |
| Thrombocytopenia | 3 (16.7) | 0 | 3 (11.1) | |
| Rash maculopapular | 3 (16.7) | 0 | 3 (11.1) | |
| Nausea | 2 (11.1) | 1 (11.1) | 3 (11.1) | |
| Nasopharyngitis | 1 (5.6) | 2 (22.2) | 3 (11.1) | |
| Lymphopenia | 1 (5.6) | 2 (22.2) | 3 (11.1) | |
| Grade ≥ 3 | 1 (5.6) | 2 (22.2) | 3 (11.1) | |
| Diarrhea | 2 (11.1) | 0 | 2 (7.4) | |
| Urinary tract infection | 2 (11.1) | 0 | 2 (7.4) | |
| Pruritus | 2 (11.1) | 0 | 2 (7.4) | |
| Cataract | 1 (5.6) | 1 (11.1) | 2 (7.4) | |
| Constipation | 1 (5.6) | 1 (11.1) | 2 (7.4) | |
| Pyrexia | 1 (5.6) | 1 (11.1) | 2 (7.4) | |
| Weight decreased | 1 (5.6) | 1 (11.1) | 2 (7.4) | |
| Insomnia | 1 (5.6) | 1 (11.1) | 2 (7.4) | |
| Epistaxis | 1 (5.6) | 1 (11.1) | 2 (7.4) | |
| Bronchitis | 0 | 2 (22.2) | 2 (7.4) | |
| Rhegmatogenous retinal | Grade ≥ 3 | 1 (5.6) | 0 | 1 (3.7) |
| Atypical mycobacterial | Grade ≥ 3 | 1 (5.6) | 0 | 1 (3.7) |
| Erythema multiforme | Grade ≥ 3 | 1 (5.6) | 0 | 1 (3.7) |
| Rash erythematous | Grade ≥ 3 | 1 (5.6) | 0 | 1 (3.7) |
| Type 2 diabetes mellitus | Grade ≥ 3 | 0 | 1 (11.1) | 1 (3.7) |
Adverse events observed in more than 2 patients and those with grade ≥ 3 were listed.
One patient discontinued tirabrutinib administration due to the worsening of an atypical mycobacterial infection at day 57 (grade 3), which resolved after discontinuation. AEs leading to study drug interruption were observed in 7 patients (38.9%) in Cohort A and 5 patients (55.6%) in Cohort B and included skin‐related AEs in 5 patients and neutropenia in 2 patients. No noticeable symptoms associated with the primary disease, with the exception of a transient increase in IgM levels in 3 (25%) of the 12 patients, were observed during the study drug interruption. Upon resuming administration of the study drug, reduced doses were administered to 6 patients (33.3%) in Cohort A and temporarily to 1 patient (11.1%) in Cohort B.
No patients exhibited IgM flares during the study drug administration. The transient increases in IgM levels observed during the study drug interruption were resolved after resuming administration. Basal serum IgA and IgG levels were mostly sustained or slightly decreased during the administration (Figure S2), and no patients required new administration of globulin preparation. Basal IgG levels in 3 patients in Cohort A were beyond the upper normal limit but decreased to within the normal limit or lower upon tirabrutinib administration. Focusing on lymphocytosis after tirabrutinib administration, 1 patient had grade 2 treatment‐related lymphocytosis (lymphocyte count: 5.5 × 109/L) at day 10, and the AE was resolved without discontinuation of tirabrutinib administration.
4. DISCUSSION
In this study, we present the results of a prospective single‐arm phase II study of tirabrutinib monotherapy in patients with treatment‐naïve and relapsed or refractory WM. We observed high ORR (96.3%) and MRR (88.9%) in all patients. The TTOR and TTMR were 0.95 and 2 mo, respectively. Although the long‐term survival could not be satisfactorily evaluated in the follow‐up period, the rapid improvement in hemoglobin levels and the gradual decrease in serum IgM levels were noted. Indeed, the hemoglobin levels increased from 10.45 g/dL at baseline to 11.95 g/dL, and the median serum IgM levels decreased from 3788 mg/dL to 2350 mg/dL after 4 wk of tirabrutinib administration in Cohort A. Approximately half of the patients (51.9%) had lymphadenopathy, and all experienced immediate reduction in lesion size or resolution. These results are comparable with those obtained for ibrutinib monotherapy in treatment‐naïve WM 19 and the rituximab‐ibrutinib group in the iNNOVATE study. 20
Our study has the limitation of tirabrutinib responses by MYD88 and CXCR4 mutational status because these mutations were assessed using different methods: MYD88 using AS‐PCR and CXCR4 using NGS. Ibrutinib monotherapy responses have been reported to be affected by MYD88 and CXCR4 mutational status, 18 , 19 whereas the responses in patients treated with ibrutinib and rituximab appeared to be independent of mutational status. 20 In the present study, 25 of 26 patients (96.2%) with genetic mutational data had MYD88L265P mutation. Although only 4 patients had CXCR4WHIM mutation, including 3 with MYD88L265P mutation, the median times to response in these patients were comparable with those of the patients with MYD88L265P/CXCR4WT.
The efficacies and safeties of various BTK inhibitors, including ibrutinib for patients with WM, are summarized in Table S3. Ibrutinib is often accompanied by AEs such as atrial fibrillation, bleeding events, and hypertension, leading many patients to discontinue treatment. 21 , 22 , 23 , 24 , 25 For instance, with ibrutinib monotherapy, 18 , 19 grade 2 or higher AEs, including atrial fibrillation (5%‐10%), bleeding events (6%‐13%), and hypertension (5%‐13%), have been reported, and 32% of patients discontinued ibrutinib treatment for any reason in relapsed or refractory WM. Mato et al. conducted a multicenter retrospective analysis of 616 patients with CLL treated with ibrutinib either commercially or in clinical trials. 22 In the report, 41% of the patients discontinued ibrutinib, with toxicity reported as the most common reason for discontinuation. The median time to ibrutinib discontinuation was 7 mo. In contrast, in the present study, tirabrutinib‐related grade 1 bleeding events occurred in 3 patients, and there were no grade 2 or higher drug‐related bleeding events. Three patients discontinued tirabrutinib during follow‐up: 1 patient discontinued because of PD after exhibiting PR, whereas the other discontinuations were not because of tirabrutinib‐related AEs. Thus, we conclude that tirabrutinib monotherapy was well tolerated, with no unexpected toxicities observed.
It is noteworthy that there were no incidents of drug‐related atrial fibrillation or hypertension in response to tirabrutinib. In contrast, a systematic review and meta‐analysis of WM and mantle cell lymphoma in addition to CLL showed that ibrutinib significantly increased the risk of both atrial fibrillation and hypertension. 23 Ibrutinib potently inhibits essential human EGFR‐related tyrosine kinases such as HER2 and HER4. 43 , 44 Conversely, tirabrutinib may not affect these tyrosine kinases. Therefore, the difference in selectivity between ibrutinib and tirabrutinib may result in different influences on cardiovascular AEs.
In the present study, 1 case had transient grade 2 lymphocytosis after tirabrutinib administration, which was resolved without any specific treatment or discontinuation of tirabrutinib. Treatment‐related lymphocytosis has not been reported as common or serious AEs in most of BTK inhibitor clinical trial for WM patients. 19 , 20 , 45 Thus, lymphocytosis accompanied by BTK inhibitor initiation in WM might not be a problematic issue, although further investigation will be required.
Second‐generation BTK inhibitors, including tirabrutinib and acalabrutinib, have been designed to have fewer off‐target effects, 21 , 28 with the goal of improving efficacy and reducing toxicity. Two reports of phase II studies of acalabrutinib monotherapy in ibrutinib‐intolerant patients for relapsed or refractory CLL are available to date, and these studies reported high response rates and satisfied safety criteria. 46 , 47 Most recently, a phase II study of acalabrutinib monotherapy for mainly patients with relapsed or refractory WM demonstrated high efficacy and feasibility, with a low discontinuation rate. 45 Considering these reports, second‐generation BTK inhibitors may contribute to reducing toxicities recognized by ibrutinib, with better dose adherence expected for patients with WM.
In summary, the present study demonstrated that tirabrutinib monotherapy is highly effective with rapid responses and is well tolerated in both treatment‐naïve patients and those with relapsed/refractory symptomatic WM. The MRR as the primary endpoint was met. However, some efficacy endpoints, such as PFS and OS, could not be evaluated because of the limited observation period. Therefore, future studies with a longer follow‐up period are warranted.
DISCLOSURE
N. Sekiguchi has received research funding from Ono Pharmaceutical. W. Munakata has received research funding from Ono Pharmaceutical. H. Handa has received research funding from Ono Pharmaceutical. H. Shibayama has received honoraria from Takeda, Novartis, Celgene, Janssen, Chugai, and Kyowa Kirin; research funding from Janssen, Ono Pharmaceutical, Celgene, Novartis, Sanofi, AstraZeneca, AbbVie, and Chugai; and scholarship endowment from Astellas, Teijin, Shionogi, Eisai, Sanofi, Taiho, and Nippon Shinyaku. Y. Terui has received honoraria from Chugai, Celgene, Bristol‐Myers Squibb, Novartis, Janssen, and Ono Pharmaceutical; and research funding from Bristol‐Myers Squibb. N. Fukuhara has received honoraria from Chugai pharma and Kyowa Kirin; and research funding from AbbVie, Bayer, Chugai, Eisai, Gilead Sciences, Incyte, Ono Pharmaceutical, and Solasia. S. Iida has received honoraria from Ono Pharmaceutical, Takeda, Janssen, Celgene, Bristol‐Myers Squibb, Daiichi Sankyo, and Sanofi; and research funding from Ono Pharmaceutical, Takeda, Bristol‐Myers Squibb, MSD, Janssen, AbbVie, Kyowa Kirin, Chugai, and Sanofi. K. Izutsu has received honoraria from Kyowa Kirin and Eisai; and research funding from Celgene, Chugai, Novartis, Ono, Pharmaceutical, Bayer, Zenyaku Kogyo, Kyowa Kirin, AstraZeneca, Eisai, Incyte, AbbVie, Symbio, Janssen, and Yakult. The other authors have no relationships to disclose. This work was supported by Ono Pharmaceutical. The study sponsor was involved in study design, writing of the report, and in the decision to submit the article for publication. Study drug was provided by Ono Pharmaceutical. All authors had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
ETHICAL CONSIDERATIONS
The institutional review board of each site approved this trial. This trial was conducted in accordance with the Declaration of Helsinki, and all patients provided written informed consent.
Supporting information
Fig S1
Fig S2
Table S1‐S3
Acknowledgments
We thank the patients participating in this trial and their supportive families. We thank Dr. Kazuo Tamura (Fukuoka University, Japan) and Dr. Hirokazu Nagai (Nagoya Medical Center, Japan) for reviewing clinical data as members of the efficacy and safety monitoring committee, and the investigators and staffs at all study sites for contributions to this trial. A medical writing support was provided, in part, by Dr. Masatoshi Esaki (Ono Pharmaceutical Co., LTD, Japan), and this study was funded by Ono Pharmaceutical Co., Ltd.
Sekiguchi N, Rai S, Munakata W, et al. A multicenter, open‐label, phase II study of tirabrutinib (ONO/GS‐4059) in patients with Waldenström’s macroglobulinemia. Cancer Sci. 2020;111:3327–3337. 10.1111/cas.14561
Data Availability Statement
Qualified researchers may request access to individual patient data through Clinical Study Data Request.com (https://www.clinicalstudydatarequest.com/). The policy for data sharing of the sponsor is available at https://www.ono.co.jp/eng/rd/policy.html.
REFERENCES
- 1. Swerdlow S, Cook J, Sohani A, et al. Lymphoplasmacytic lymphoma In: Swerdlow S, Campo E, Harris N, eds. WHO Classification of Tumour of Hematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2017:232–235. [Google Scholar]
- 2. Owen RG, Treon SP, Al‐Katib A, et al. Clinicopathological definition of Waldenstrom’s macroglobulinemia: Consensus Panel Recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin Oncol. 2003;30:110–115. [DOI] [PubMed] [Google Scholar]
- 3. Buske C, Sadullah S, Kastritis E, et al. Treatment and outcome patterns in European patients with Waldenström’s macroglobulinaemia: a large, observational, retrospective chart review. Lancet Haematol. 2018;5:e299–e309. [DOI] [PubMed] [Google Scholar]
- 4. Olszewski AJ, Treon SP, Castillo JJ. Application and Outcomes of Bendamustine‐ or Bortezomib‐Based Therapy for Waldenstrom’s Macroglobulinemia [abstract]. Blood. 2017;130(Supplement 1):348.28550042 [Google Scholar]
- 5. Olszewski AJ, Treon SP, Castillo JJ. Evolution of management and outcomes in Waldenström Macroglobulinemia: a population‐based analysis. Oncologist. 2016;21:1377–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Treon SP, Ioakimidis L, Soumerai JD, et al. Primary therapy of Waldenström macroglobulinemia with bortezomib, dexamethasone, and rituximab: WMCTG clinical trial 05–180. J Clin Oncol. 2009;27:3830–3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dimopoulos MA, Anagnostopoulos A, Kyrtsonis MC, et al. Primary treatment of Waldenström macroglobulinemia with dexamethasone, rituximab, and cyclophosphamide. J Clin Oncol. 2007;25:3344–3349. [DOI] [PubMed] [Google Scholar]
- 8. Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first‐line treatment for patients with indolent and mantle‐cell lymphomas: An open‐label, multicentre, randomised, phase 3 non‐inferiority trial. Lancet. 2013;381:1203–1210. [DOI] [PubMed] [Google Scholar]
- 9. Dimopoulos MA, Kastritis E. How I treat Waldenström’s Macroglobulinemia. Blood. 2019;134:2022–2035. [DOI] [PubMed] [Google Scholar]
- 10. Qiu Y, Kung H‐J. Signaling network of the Btk family kinases. Oncogene. 2000;19:5651–5661. [DOI] [PubMed] [Google Scholar]
- 11. Kurosaki T, Hikida M. Tyrosine kinases and their substrates in B lymphocytes. Immunol Rev. 2009;228:132–148. [DOI] [PubMed] [Google Scholar]
- 12. Genevier HC, Hinshelwood S, Gaspar HB, et al. Expression of Bruton’s tyrosine kinase protein within the B cell lineage. Eur J Immunol. 1994;24:3100–3105. [DOI] [PubMed] [Google Scholar]
- 13. Ponader S, Burger JA. Bruton’s tyrosine kinase: From X‐linked agammaglobulinemia toward targeted therapy for B‐cell malignancies. J Clin Oncol. 2014;32:1830–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davis RE, Ngo VN, Lenz G, et al. Chronic active B‐cell‐receptor signalling in diffuse large B‐cell lymphoma. Nature. 2010;463:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. N Engl J Med. 2012;367:826–833. [DOI] [PubMed] [Google Scholar]
- 16. Treon SP, Hunter ZR. A new era for Waldenstrom macroglobulinemia: MYD88 L265P. Blood. 2013;121:4434–4436. [DOI] [PubMed] [Google Scholar]
- 17. Yang G, Zhou Y, Liu X, et al. A mutation in MYD88 (L265P) supports the survival of lymphoplasmacytic cells by activation of Bruton tyrosine kinase in Waldenström macroglobulinemia. Blood. 2013;122:1222–1232. [DOI] [PubMed] [Google Scholar]
- 18. Treon SP, Tripsas CK, Meid K, et al. Ibrutinib in previously treated Waldenström’s macroglobulinemia. N Engl J Med. 2015;372:1430–1440. [DOI] [PubMed] [Google Scholar]
- 19. Treon SP, Gustine J, Meid K, et al. Ibrutinib monotherapy in symptomatic, treatment‐naïve patients with waldenström macroglobulinemia. J Clin Oncol. 2018;36:2755–2761. [DOI] [PubMed] [Google Scholar]
- 20. Dimopoulos MA, Tedeschi A, Trotman J, et al. Phase 3 trial of Ibrutinib plus rituximab in Waldenstrom’s macroglobulinemia. N Engl J Med. 2018;378:2399–2410. [DOI] [PubMed] [Google Scholar]
- 21. Stephens DM, Byrd JC. How I manage ibrutinib intolerance and complications in patients with chronic lymphocytic leukemia. Blood. 2019;133:1298–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mato AR, Nabhan C, Thompson MC, et al. Toxicities and outcomes of 616 ibrutinib‐treated patients in the united states: A real‐world analysis. Haematologica. 2018;103:874–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Caldeira D, Alves D, Costa J, Ferreira JJ, Pinto FJ. Ibrutinib increases the risk of hypertension and atrial fibrillation: Systematic review and meta‐analysis. PLoS One. 2019;14:e0211228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barr PM, Brown JR, Hillmen P, et al. Impact of ibrutinib dose adherence on therapeutic efficacy in patients with previously treated CLL/SLL. Blood. 2017;129:2612–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yun S, Vincelette ND, Acharya U, Abraham I. Risk of atrial fibrillation and bleeding diathesis associated with ibrutinib treatment: a systematic review and pooled analysis of four randomized controlled trials. Clin. Lymphoma Myeloma Leuk. 2017;17:31–37.e13. [DOI] [PubMed] [Google Scholar]
- 26. Liclican A, Serafini L, Xing W, et al. Biochemical characterization of tirabrutinib and other irreversible inhibitors of Bruton’s tyrosine kinase reveals differences in on ‐ and off ‐ target inhibition. Biochim BiophysActa Gen Subj. 2020;1864:129531. [DOI] [PubMed] [Google Scholar]
- 27. Kozaki R, Vogler M, Walter H, et al. Responses to the selective Bruton’s Tyrosine Kinase (BTK) inhibitor tirabrutinib (ONO/GS‐4059) in diffuse large B‐cell lymphoma cell lines. Cancers (Basel). 2018;10:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Owen C, Berinstein NL, Christofides A, Sehn LH. Review of Bruton tyrosine kinase inhibitors for the treatment of relapsed or refractory mantle cell lymphoma. Curr Oncol. 2019;26:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Walter HS, Rule SA, Dyer MJS, et al. A phase 1 clinical trial of the selective BTK inhibitor ONO/GS‐4059 in relapsed and refractory mature B‐cell malignancies. Blood. 2016;127:411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Munakata W, Ando K, Hatake K, et al. Phase I study of tirabrutinib (ONO‐4059/GS‐4059) in patients with relapsed or refractory B‐cell malignancies in Japan. Cancer Sci. 2019;110:1686–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kyle RA, Treon SP, Alexanian R, et al. Prognostic markers and criteria to initiate therapy in Waldenstrom’s macroglobulinemia: Consensus Panel Recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin Oncol. 2003;30:116–120. [DOI] [PubMed] [Google Scholar]
- 32. Dimopoulos MA, Kastritis E, Owen RG, et al. Treatment recommendations for patients with Waldenström macroglobulinemia (WM) and related disorders: IWWM‐7 consensus. Blood. 2014;124:1404–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morel P, Duhamel A, Gobbi P, et al. International prognostic scoring system for Waldenström macroglobulinemia. Blood. 2009;113:4163–4170. [DOI] [PubMed] [Google Scholar]
- 34. Jiménez C, del Chillón MC, Balanzategui A, et al. Detection of MYD88 L265P mutation by real‐time allele‐specific oligonucleotide polymerase chain reaction. Appl Immunohistochem Mol Morphol. 2014;22:768–773. [DOI] [PubMed] [Google Scholar]
- 35. Rothberg JM, Hinz W, Rearick TM, et al. An integrated semiconductor device enabling non‐optical genome sequencing. Nature. 2011;475:348–352. [DOI] [PubMed] [Google Scholar]
- 36. Hunter ZR, Xu L, Yang G, et al. The genomic landscape of Waldenström macroglobulinemia is characterized by highly recurring MYD88 and WHIM‐like CXCR4 mutations, and small somatic deletions associated with B‐cell lymphomagenesis. Blood. 2014;123:1637–1646. [DOI] [PubMed] [Google Scholar]
- 37. Treon SP, Cao Y, Xu L, et al. Somatic mutations in MYD88 and CXCR4 are determinants of clinical presentation and overall survival in Waldenström macroglobulinemia. Blood. 2014;123:2791–2796. [DOI] [PubMed] [Google Scholar]
- 38. Owen RG, Kyle RA, Stone MJ, et al. Response assessment in Waldenström macroglobulinaemia: update from the VIth International Workshop. Br J Haematol. 2013;160:171–176. [DOI] [PubMed] [Google Scholar]
- 39. Abeykoon JP, Zanwar S, Ansell SM, et al. Ibrutinib monotherapy outside of clinical trial setting in Waldenström macroglobulinaemia: practice patterns, toxicities and outcomes. Br J Haematol. 2020;188:394–403. [DOI] [PubMed] [Google Scholar]
- 40. Gertz MA, Rue M, Blood E, et al. Multicenter Phase 2 Trial of Rituximab for Waldenström Macroglobulinemia (WM): An Eastern Cooperative Oncology Group Study (E3A98). Leuk Lymphoma. 2004;45:2047–2055. [DOI] [PubMed] [Google Scholar]
- 41. Treon SP, Hunter ZR, Matous J, et al. Multicenter clinical trial of bortezomib in relapsed/refractory Waldenstrom’s macroglobulinemia: Results of WMCTG trial 03–248. Clin Cancer Res. 2007;13:3320–3325. [DOI] [PubMed] [Google Scholar]
- 42. Leblond V, Johnson S, Chevret S, et al. Results of a randomized Trial of Chlorambucil versus Fludarabine for patients with untreated Waldenström Macroglobulinemia, marginal zone lymphoma, or lymphoplasmacytic lymphoma. J Clin Oncol. 2013;31:301–307. [DOI] [PubMed] [Google Scholar]
- 43. Lee K‐F, Simon H, Chen H, et al. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature. 1995;378:394–398. [DOI] [PubMed] [Google Scholar]
- 44. Gassmann M, Casagranda F, Orioli D, et al. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378:390–394. [DOI] [PubMed] [Google Scholar]
- 45. Owen RG, McCarthy H, Rule S, et al. Acalabrutinib monotherapy in patients with Waldenström macroglobulinemia: a single‐arm, multicentre, phase 2 study. Lancet Haematol. 2020;7:e112–e121. [DOI] [PubMed] [Google Scholar]
- 46. Awan FT, Schuh A, Brown JR, et al. Acalabrutinib monotherapy in patients with chronic lymphocytic leukemia who are intolerant to ibrutinib. Blood Adv. 2019;3:1553–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rogers KA, Thompson PA, Allan JN, et al. Phase 2 study of acalabrutinib in ibrutinib (IBR)‐intolerant patients (pts) with relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL). [abstract]. J. Clin. Oncol. 2019;37(15_suppl):7530. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Table S1‐S3
Data Availability Statement
Qualified researchers may request access to individual patient data through Clinical Study Data Request.com (https://www.clinicalstudydatarequest.com/). The policy for data sharing of the sponsor is available at https://www.ono.co.jp/eng/rd/policy.html.


