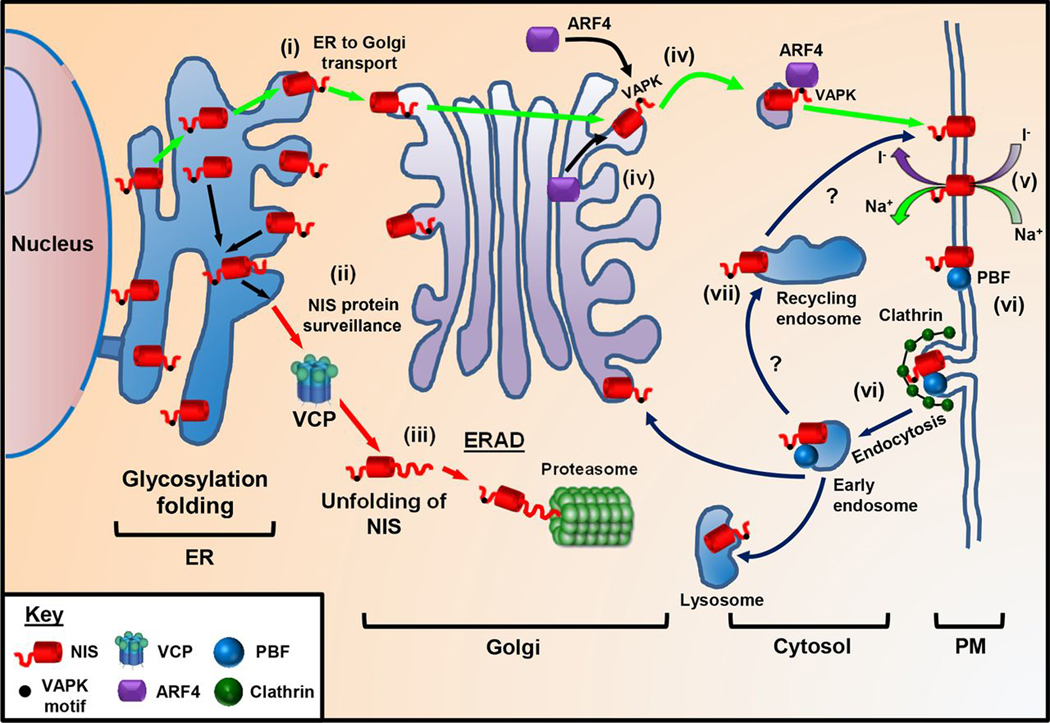
Figure 7.
Putative model of NIS trafficking. NIS maintains a delicate balance between protein synthesis, folding, assembly, trafficking, and degradation. (i) We propose NIS is glycosylated in the ER and upon correct folding transported to the Golgi. (ii) Protein surveillance pathways exist that target NIS for ERAD. As VCP does not require ATPase activity to inhibit NIS function, it is likely VCP acts to unfold NIS (iii) prior to proteasomal degradation. (iv) ARF4 recognizes the VAPK motif in the NIS C-terminus and promotes vesicular trafficking to the PM, where NIS is active (v). (vi) PBF has a YARF endocytosis motif and acts to bind and internalize NIS away from the PM in a clathrin-dependent process. (vii) Although inhibition of recycling by dynasore suggests that ARF4 shuttles NIS to the PM, other proteins must promote recycling of NIS to the PM as with most PM transporters.