Fig. 4.
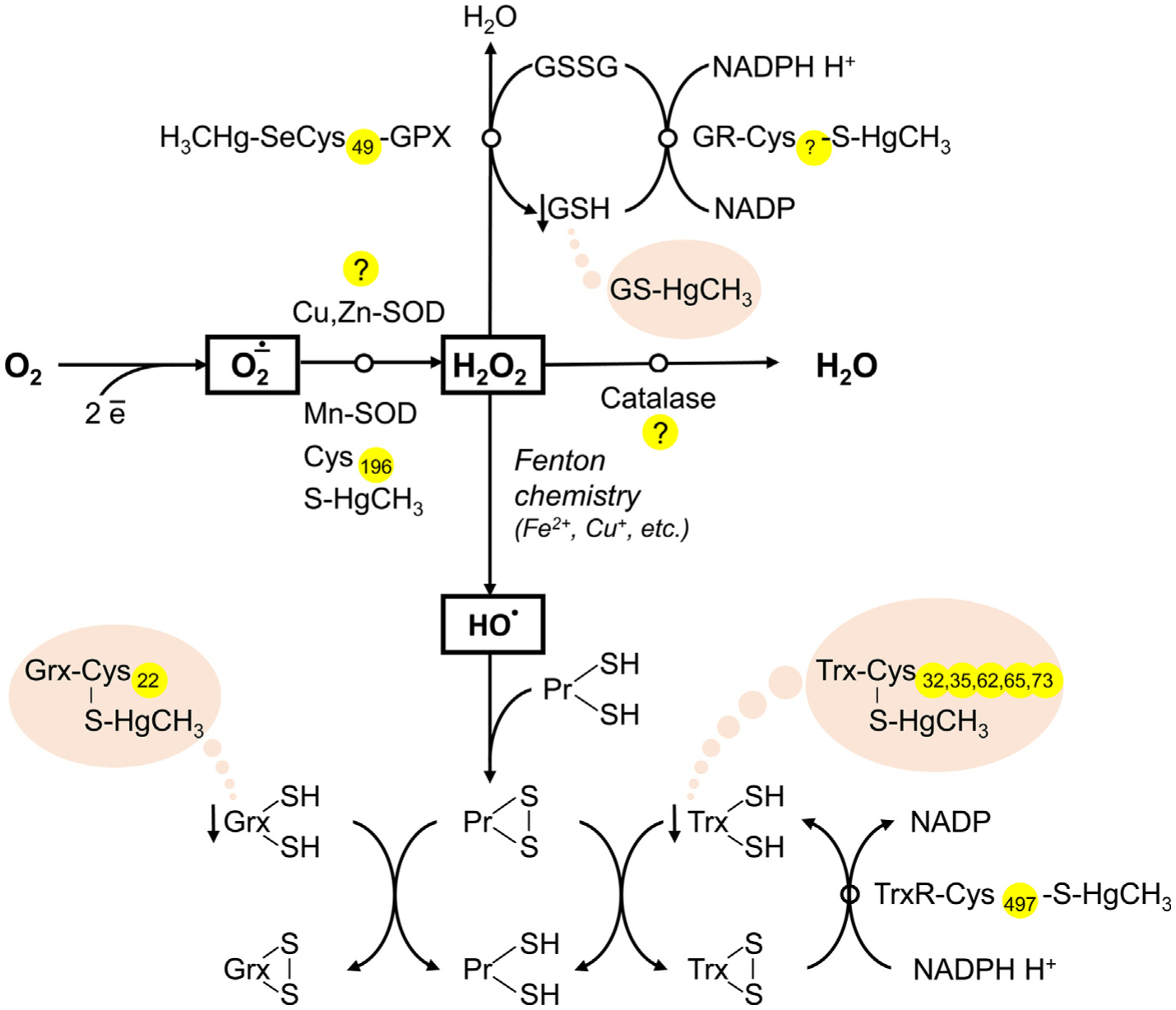
The role of Hg-SH binding in Hg-induced decrease in antioxidant system activity. Mercury was shown to inhibit antioxidant enzymes, including Cu,Zn-SOD, Mn-SOD, catalase, GPX, GR, and TrxR, although the particular role of Hg-SH interaction in this process is unclear. Inhibition of Mn-SOD was shown to be associated with Hg-SH binding at Cys196, whereas the particular location of Hg-binding cysteine residue in GR is not estimated. Inactivation of GPX may occur due to Hg binding to SeCys49 residue. In turn, recent data demonstrate that direct Hg-SH interaction is unlikely to be involved in inhibition of Cu,Zn-SOD, and catalase. In addition to Hg-induced antioxidant enzyme inactivation, Hg may also bind thiol groups of proton-donor cofactors (GSH, Trx, Grx), thus limiting their availability for reduction.
