Abstract
Introduction
Dermatophytosis caused by Nannizzia gypsea formerly Microsporum gypse um is rare in occurrence due to its geophilic adaptation and weak pathogenic potential in establishing infection in humans. The taxonomical status of N. gypsea has been controversial over the years and has now reached a concordance among mycologists. Innumerable reports of N. gypsea causing widespread infection in human immunodeficiency virus patients trails them as an important agent of consideration in an immunocompromised host. There have been sporadic reports of N. gypsea causing glabrous skin tinea and onychomycosis in healthy patients and the prevalence reports gravitate around 1–6.5 %. A variety of non-anthropophilic dermatophytes including novel species have now been implicated in causing dermatophytosis reflecting the era of crux changes in the epidemiology.
Case report
We present a case of chronic dermatophytosis in a 22-year-old healthy Indian with a history of contact with a dog and soil and other factors favouring dermatophytosis. Conventional and molecular sequencing established the isolate as N. gypsea. Antifungal susceptibility test revealed a higher MIC of griseofulvin and lower MIC to azoles and terbinafine. The patient had complete clinical resolution following administration of oral terbinafine.
Conclusion
Amidst the hyper-endemic-like scenario of tinea in India, this case report stands as a unique example of a patient infected with N. gypsea showing complete clinical resolution using terbinafine. Studies implicating N. gypsea in an immunocompetent host are rare and there is a need for more studies on geophilic dermatophytes causing tinea in the man for laying down effective preventive measures.
Keywords: Nannizzia gypsea, geophile, tinea corporis et cruris, terbinafine, ITS sequencing, CLSI M38A2, immunocompetent host
Introduction
Superficial cutaneous mycoses are principally comprised of dermatophytosis and other dermatomycoses like pityriasis versicolor, candidiasis and rare infections like tinea nigra and Piedra. Dermatophytosis is caused by a group of dermatophytes which were classically circumscribed into three main genera Trichophyton, Epidermophyton and Microsporum based on their morphology of macroconidia [1]. The taxonomical status of family Arthrodermataceae, which comprise the dermatophytes and congeners has come to an acceptable level of concordance [1]. Molecular characterization of dermatophytes using the sequences of gene regions of Internal transcribed spacer (ITS) of ribosomal DNA, partial large subunit (LSU), partial β-tubulin(TUB), and 60S ribosomal protein L10 proposes a new taxonomy which revises the existing three genera to nine- genus classification—Trichophyton, Microsporum, Epidermophyton (Classical three-genera) Nannizzia, Lophophyton, Arthroderma, Ctenomyces, Guarromyces, and Paraphyton (six-new genera) [1]. Furthermore, based on host preference and the natural habitat, dermatophytes are grouped as the anthropophilic species, which predominantly infect humans, geophilic species that are soil loving and may rarely infect both humans and animals, and zoophilic species, which generally infect non-human mammals and have been frequently implicated in causing infections in humans, respectively. Human infections caused by Nannizzia gypsea formerly Microsporum gypse um are comparatively rare due to their geophilic nature, which suggests that they have a natural resistance to infection in humans [1, 2]. A variety of non-anthropophilic dermatophytes like Nannizzia aenigmaticum, Paraphyton mirabile, Arthroderma eboreum, Arthroderma onychocola and geophilic species like Nannizzia nana, Nannizzia incurvata, Nannizzia fulva, and Microsporum racemosum (Fac. syn. Paraphyton cookie) which were never reported to cause human infections in the past have been now implicated in causing dermatophytosis reflecting an era of crux changes in the epidemiology of tinea [1, 3].
There is a geographical and temporal variation in the incidence of dermatophytes causing tinea around the world. N. gypsea is a geophilic and keratinophilic fungus distributed worldwide. Several states from India and neighbouring countries like Nepal have documented the habitat of this fungus along with other keratinophilic fungi from the soil and the immediate premises [4]. These fungi have a special predilection towards black chernozemic soil, which usually has high moisture and neutral pH [5]. Prevalence of N.gypsea from the global literature gravitate around 1–6.5 %. In India, the highest rate of 6.5 % has been reported from Jaipur, Rajasthan followed by Shimla, Himachal Pradesh [6, 7]. There is a vacuum in systematic prevalence studies on N. gypsea causing human infections. From the classical circumscriptions of M. gypseum complex it is not possible to distinguish among the species of M.gypseum, M. fulvum, and M. incurvatum based on the morphological features alone [6]. With the introduction of molecular methods, the identification of dermatophytes to the species-level was achieved which in turn has led to a rise in reports of rare geophilic dermatophytes causing human infections, for example, in 2018, two novel species of Nannizzia were reported—Nannizzia perplicata sp. nov. and N.graeserae sp. nov. [8, 9]. Earlier reported studies were incomplete as they used only conventional identification methods. Therefore, it is a matter of debate whether these infections are rarely reported or never identified accurately and thus their true incidence is underestimated [6].
In India, there has been an increase in the prevalence of chronic and recurrent dermatophytosis over last 4–5 years [10]. Treating patients with dermatophytosis has become a challenge to the dermatologists due to an upsurge in the refractory and recalcitrant entities associated with treatment failures [10, 11]. Therefore, identification of etiological agents and confirmation of diagnosis, information on in vitro antifungal susceptibilities for commonly used antifungal agents in clinical settings is both relevant in treatment with precision and to address the current epidemiological concerns. Drug resistance in dermatophytes is not a common entity at present, but few studies from India have reported putative genes and point mutations conferring resistance to terbinafine in Trichophyton rubrum and Trichophyton interdigitale [11]. The present rare case describes the involvement of N. gypsea in chronic tinea corporis et cruris in an immunocompetent cook. The isolate was confirmed using molecular sequencing and antifungal susceptibility pattern revealed higher MICs of griseofulvin and lower MICs of terbinafine. The patient was successfully treated with oral-terbinafine and there has been no recurrence in the follow-up period of 1 year.
Case Report
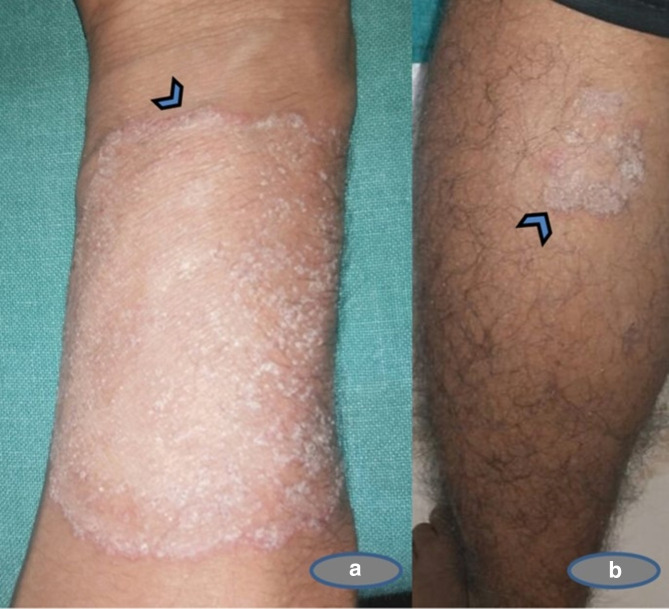
A 22-year-old male patient, cook by occupation, presented to the dermatology outpatient department (OPD) with a history of itchy skin lesions over the body, forearms and legs for 7 months. Lesions initially started during summer as a small papule and eventually progressed to form large circinate plaques. General and systemic examination of the patient was unremarkable. On dermatological examination, well-defined circinate, scaly plaques with erythematous papules of varying sizes were present over the flexor aspect of the right forearm and lower left leg, as shown in Fig. 1a, b.
Fig. 1.
Description of lesions of Tinea in an immunocompetent Indian male. (a) Circinate scaly plaques with a well-defined erythematous scaly plaque of size 5x6 cm with papules in the border on the flexor aspect of the right forearm (arrowhead); (b) Erythematous irregular scaly plaques over the lower part of the lower leg of size 2x2 cm (arrowhead).
On further examination, similar lesions were seen in the lower abdomen, groin and the gluteal region (not shown in Fig. 1). His palms, soles, hair and nails were normal without any concomitant infection. The patient denied a history of any application of topical corticosteroids or any self-medication. He gave a history of contact with black soil while working in resorts and continuous exposure to a warm and humid temperature in the cooking area. He shared his room and linen with two other occupants, none of whom had a history of similar lesions. The patient also informed about contact with a pet dog. There was no history of any other co-morbidities. His serological tests for hepatitis B and human immunodeficiency virus were negative.
Processing of the specimen for mycological examination
After wiping the lesions with spirit, skin scrapings were collected from the active borders of the lesions from the right forearm, thigh and the lower abdomen and were subjected to 20 % potassium hydroxide (KOH) mount. The rest of the specimen was inoculated on Sabouraud’s dextrose agar (SDA) with 0.004 % chloramphenicol and 0.05 % cycloheximide (HiMedia, India) and a screening medium Dermatophyte test medium (DTM) agar base with 'Dermato Supplement', which includes cycloheximide, chlortetracycline and gentamicin (HiMedia, India) and incubated for 2–4 weeks in ambient air at 28 °C in a Biological Oxygen Demand/Biochemical Oxygen Demand (BOD) incubator.
Identification of the isolate using morphological and phenotypic features
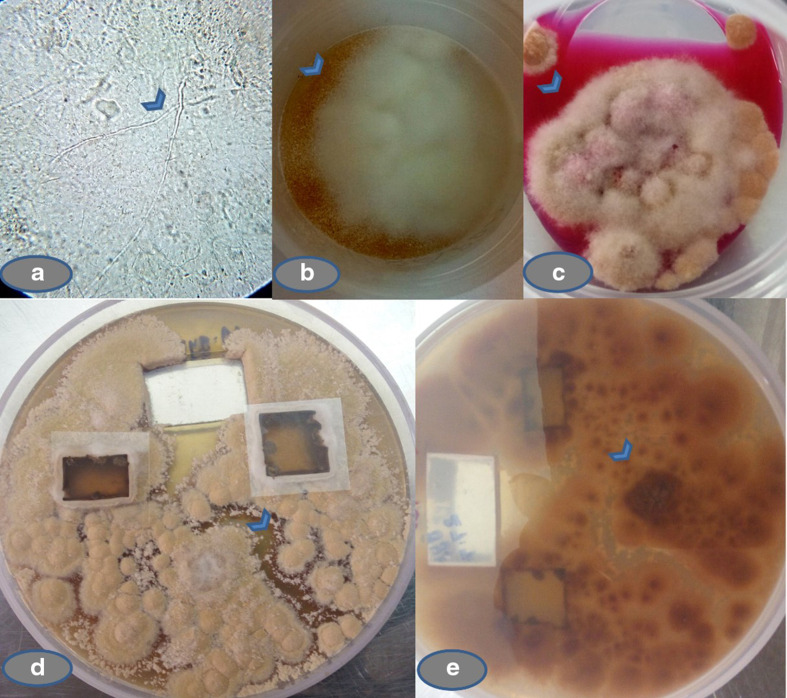
Direct microscopic examination of skin scrapings from multiple sites on 20 % KOH mount revealed thin hyaline septate branching hyphae characteristic of dermatophytes. Growth on SDA initially was cottony, colonies matured within two-weeks showing powdery growth. On DTM, colonies grew within 5–7 days and turned the medium into red colour by changing the pH. When sub-cultured on to potato dextrose agar (PDA), a rapid buff or brownish colour growth with powdery surface texture, and peripheral fringe of a white zone with a reddish reverse pigment was seen within 2 weeks of incubation. On further incubation, the colonies turned pleomorphic with ragged edges, as shown in Fig. 2a–e.
Fig. 2.
Direct microscopy and fungal culture of the skin scrapings. (a) Direct 20 % KOH preparation of the skin scrapings (40X) showing thin hyaline septate hyphae characteristic of dermatophytes (arrowhead). (b) Growth on SDA with chloramphenicol and cycloheximide showing powdery growth (arrowhead). (c) Growth on DTM with Dermato Supplement changing pH (arrowhead) of the media. (d) Subculture on PDA after 2 weeks showing powdery surface texture with buff or brownish colour and peripheral fringe of a white zone (arrowhead). (e) Reddish reverse on PDA (arrowhead).
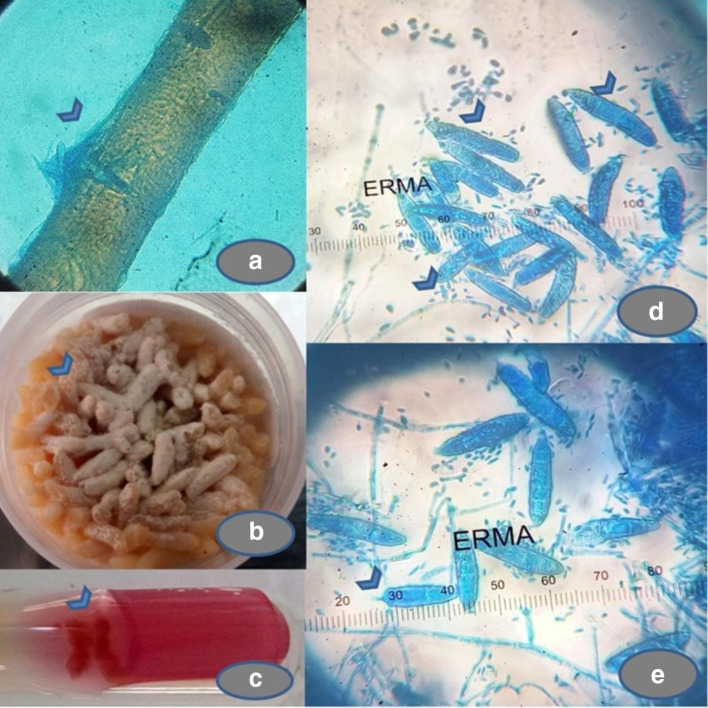
Microplate cultures and rapid adhesive cellophane impression mounts were performed along with the physiological tests for the identification of the dermatophyte. The isolate perforated in vitro sterile pre-pubescent hair, and hydrolysed urease within the 10-days of incubation at room temperature. Lacto phenol cotton blue (LPCB) mounts revealed a moderate number of microconidia. Macroconidia were initially sparse but, older cultures showed an increased number of macroconidia, which are spindle-shaped, thin-walled with echinulations and blunt tips, as shown in Fig. 3a–e. The above morphological and physiological features concluded the phenotypic identification as M. gypse um. Due to the sparse production of macroconidia on initial subcultures on SDA and PDA, and its rarity in isolating from an immunocompetent host, the isolate was submitted for molecular confirmation and antifungal susceptibility pattern to the National Culture Collection of Pathogenic Fungi (NCCPF), Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India with a submission ID IL_2795 (Myc_186).
Fig. 3.
Physiological and morphological features of the isolate. (a) Perforating organs on sterile pre-pubescent hair in vitro (arrowhead). (b) Powdery growth with brownish pigment on sterile polished rice grain inoculation (arrowhead). (c) Hydrolysis of Christensen’s urea medium with 10 days of inoculation (arrowhead). (d, e) Lacto phenol cotton blue (LPCB) mount showing a moderate number of teardrop shaped microconidia (arrowheads), measuring 2–3×5–8 µm smooth walled and are borne laterally on the septate hyphae, arranged in solitary and small groups were seen. Numerous spindle-shaped macroconidia (arrowheads), thin-walled and echinulate surface (arrowheads) with 4–6 septa (arrowheads) and measuring 150×15 µm (arrowhead) and a few of them showing blunt tips (arrowhead).
Confirmation of Nannizzia gypsea by molecular identification
Molecular identification of the culture was performed at the NCCPF, PGIMER, Chandigarh, India, by sequencing the ITS region of the rDNA using the universal primer pair ITS-1(5′-TCCGTAGGTGAACCTTGCGG-3′) and ITS-4 (5′-TCCTCCGCTTATTGATATGC-3′) [11]. Mycelium was gently scraped with a sterile scalpel from 10-days old pure culture on PDA, and is transferred in to a sterile mortar and grounded in the presence of liquid nitrogen and 1 ml of extraction buffer (0.2 mol l−1 Tris-HCl, 10 mmol l−1 EDTA, 0.5 mol l−1 NaCl, 1 % SDS) and crushed by a pestle to form a slurry. DNA extraction was performed by phenol: chloroform: isoamyl alcohol (25:24:1) and ethanol precipitation method. The quantity of DNA was assessed by using NanoDropspectrophotometer reading (Thermo Scientific) and gel electrophoresis on 1% agarose gel. Amplification of ITS region was performed inthe PCR assay and the purified products of ITS PCR was processed for sequencing using universal primers. The ITS sequencing was carried out using ABI PRISM BigDye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems, Foster City, CA, USA), according to the manufacturers’ recommendations, sequencing and analysis was performed in automated DNA sequencer-ABI Prism 3130XL Genetic Analyzer (Applied Biosystems) [11].
N. gypsea was confirmed by comparing the sequence with ITS sequence database in the Centraalbureau voor Schimmelcultures (CBS), Utrecht, the Netherlands, and the sequence showed 100% similarity with the type strain CBS161.69 N. gypsea [12].The isolate was confirmed as N. gypsea by ITS sequencing and was deposited in GenBank ITS databases and published in the NCBI database on 6/8/2018 with accession number MH715973.1 and name Nannizzia gypsea strain IL2795_Myc_186.
In vitro antifungal susceptibility testing (AFST)
Antifungal susceptibility testing (AFST) for the isolate was performed at NCCPF, PGIMER, Chandigarh, India using the standardized broth microdilution (BMD) method according to the Clinical Laboratory Standards Institute (CLSI) document M38A2. The five antifungal drugs widely used in the treatment of dermatophytosis were tested, which includes itraconazole (ITR), sertaconazole (SER), terbinafine (TER), griseofulvin (GRI) and amorolfine (AMO). All the antifungal drugs tested were of reagent-grade powders (Sigma-Aldrich, Bengaluru, India) and were directly procured by the NCCPF, Chandigarh, India. The inoculum was prepared using RPMI 1640 (HiMedia, India) with l-glutamine and without bicarbonate. Antimycotic drugs were diluted using 0.165 M morpholine propane sulfonic acid (MOPS) (Sigma-Aldrich Chemie GmbH, Germany) with pH adjusted at 7.0. The following quality control strains were used: Candida krusei ATCC 6258, Aspergillus flavus ATCC 20430 and Candida parapsilosis ATCC 22019. Broth microdilution using 96-welled microtitre plates were used. Preparation of inoculum and dilutions were performed as described by Rudramurthy et al. [11]. The antifungal drug dilutions stored at −80 °C prior to use. The results of the antifungal susceptibility testing of five antifungal agents on N. gypsea are shown in Table 1.
Table 1.
Results of AFST in vitro of Nannizzia gypsea (IL2795_Myc 186) from Tinea corporis et cruris from a healthy Indian male.
|
Antifungal tested |
Dilution range in µg ml−1 |
MIC in µg ml−1 |
|---|---|---|
|
Amorolfine (AMO) |
0.0078–4 µg ml−1 |
0.0156 |
|
Itraconazole (ITR) |
0.016–16 µg ml−1 |
0.0625 |
|
Sertaconazole(SER) |
0.016–16 µg ml−1 |
0.0312 |
|
Terbinafine (TER) |
0.004–4 µg ml−1 |
0.0156 |
|
Griseofulvin (GRI) |
0.25–128 µg ml−1 |
16 |
AFST results of N. gypsea showed lower MIC of AMO 0.0156 µg ml−1, TER 0.0156 µg ml−1, SER 0.0312 µg ml−1, ITR 0.0625 µg ml−1 and higher MIC of GRI 16 µg ml−1.
Antifungal therapy and follow-up
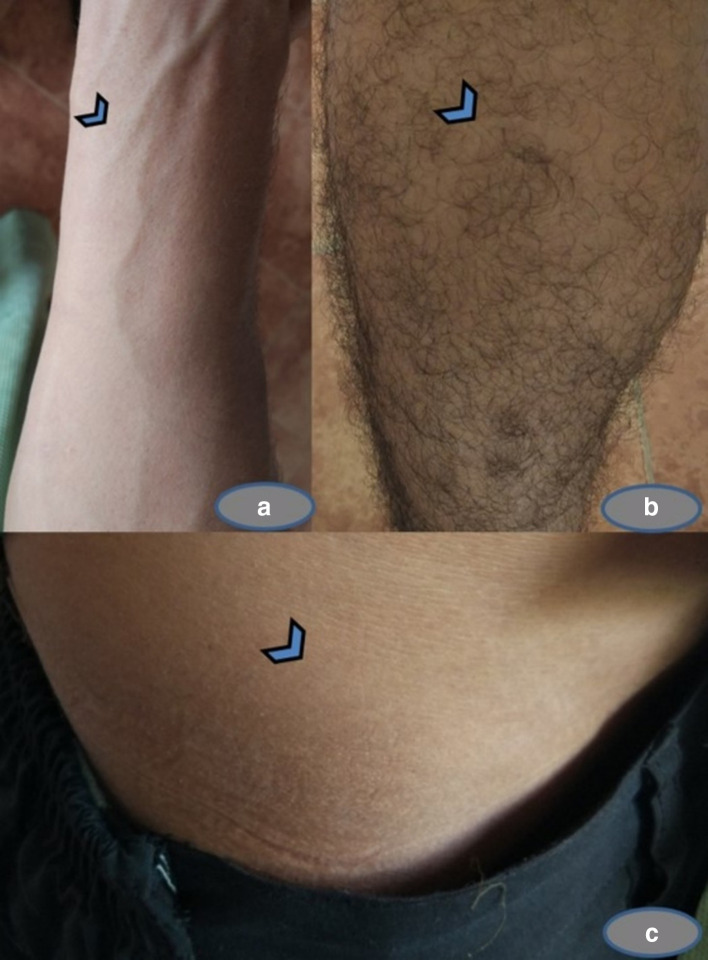
The patient was started on a combination of oral terbinafine 250 mg once daily at bedtime for 3 weeks, and topical 1 % luliconazole applied once daily for 3 weeks. The patient was advised not to spend more time in the kitchen and avoid humid premises and to maintain general hygiene with twice a day bathing and washing and drying the clothes regularly. He was also advised not to get in contact with the dog and restrict the exposure to soil. The patient did not turn up for a follow-up and hence due to the rarity of isolate we made a visit to the place where the patient worked to obtain the post-treatment information. Obtaining specimen from the dog was unsuccessful at the time of visit. Upon eliciting further, the patient gave the history of complete resolution of lesions after oral terbinafine 250 mg once daily bedtime for 3 weeks and he admitted to having discontinued the topical antifungal cream 1 % luliconazole after 5 days of use. His itching and inflammatory lesions resolved without any residual scars or relapse after 1-year post-treatment, as shown in Fig. 4a–c.
Fig. 4.
Follow-up of tinea owing to N. gypsea after successful terbinafine regimen. Complete resolution of tinea caused by N. gypsea on the flexor aspect (a) of the right forearm, lower leg (b), and the lower abdomen (c) and other sites after terbinafine therapy without any residual scar or relapse at the follow-up of 1-year duration.
Discussion
Demonstration of hyphae in skin scrapings has been considered as the presumptive diagnosis of tinea, but in respect to geophilic dermatophytes, many studies have reported only one-third of the skin scrapings being positive for fungal elements on direct microscopy using KOH [6]. The skin scrapings from multiple sites from the present case were positive for hyaline septate hyphae on KOH mount.
The true incidence of geophile N. gypsea is underestimated since it shares the morphological features of other members of N. gypsea complex. There is a geographical and temporal variation of the prevalence of geophilic dermatophyte like N. gypsea. This heterogeneity in the distribution of etiological agents in different parts of the world and from one geographic region to another region in a country has been attributed to various socio-demographic, environmental, clinical and mycological factors. Due to the vacuum in the systematic prevalence studies on N. gypsea, the reported data are not species specific as the identification of the clinical isolates was in majority made by conventional identification methods. Therefore, it is a matter of debate, whether these infections are rarely reported or never identified accurately [6]. Molecular applications in mycology like ITS sequencing of rDNA or beta-tubulin gene have paved the way to distinguish the species of Nannizzia.
There was a considerable discordance in the classification of Nannizzia and Microsporum among the mycologists. In as early as 1927, Nannizzi was the first to describe the teleomorphic states of M. gypseum, which was largely ignored by the scientific community. The basis of the classification of anamorph species of Microsporum was based on the characteristically rough-walled, blunt, club-shaped, multi-celled macroconidia [13]. The work met with concordance only after the descriptions of anamorph state of Arthroderma incurvatum and the teleomorph state of Trichophyton ajelloi by Dawson and Gentles. Furthermore, Griffin and Stockdale differed with findings of Nannizzi and elucidated that M. gypse um and N. incurvatum exists in two teleomorphic states and named N. gypsea as the anamorph. Shortly after this, Arthroderma was retained exclusively for naming the teleomorphic state and Nannizzia and Microsporum for describing the anamorphic states, respectively [14].
Multiple molecular markers along with ITS region of rDNA, BT2 (beta tubulin 2 gene), TEF1 (Translation elongation factor 1-α gene), partial LSU (large subunit), calmodulin gene, S60 and L10 are extensively used for distinguishing the genera of dermatophytes [1]. The proposed new dermatophyte taxonomy restricts genus Microsporum to only three-species principally causing human infections which are M. audouinii,M.canis, and M. ferrugineum, whereas the rare geophilic and zoophilic species that cause human infections are placed in the newly classified six genera [1]. Some of the species, which were formerly classified in the genus Microsporum, are now included in genera Nannizzia revising the list to nine species, which includes N. aenygmaticum, N. corniculata, N. duboisii, N. fulva, N. gypsea, N. incurvata, N. nana, N. persicolor and N. praecox [1]. Furthermore, extensive taxonomical revisions are underway taking Arthrodermatacea as a perspective. Several new species have been designated by mating experiments in both geophilic and zoophilic dermatophytes with a few of them require exact borderlines to distinguish among other species, and a plethora of other dermatophytes are yet to be elucidated from various under sampled habitats and this is an active area of consideration in dermatophyte research [1]. Two novel species of Nannizzia have been reported from the United Kingdom and India in 2018. The isolate from the United Kingdom was from a tinea corporis in a 44-year-old Caucasian woman and phylogenetic analysis showed that the isolate was distantly related to sister species of Nannizzia nana and is named as Nannizzia perplicata sp. nov. [8]. The Indian isolate was from a vicinity of a barbershop which showed a maximum similarity with teleomorph of N. corniculata and is now named as a new geophile N. graeserae sp.nov. using morphological and ITS sequence divergence studies [9]. The present isolate from immunocompetent adult was confirmed as N. gypsea using ITS sequencing and was submitted in GenBank, comparison of the sequence showed 100% similarity with reference ITS type materials of N. gypsea.
N. gypsea in the present case was isolated from an immunocompetent cook who had a history of contact with soil and a dog and other favourable factors and therefore it was difficult to ascertain the source of this isolate. Determination of actual adaptation of geophilic and zoophilic dermatophytes is complex because the species, which are principally designated as geophilic ex: M. gypseum complex, are inhabitants of soil and predominately dwell both in soil and fur of apparently healthy animals. Ecological studies by Rippon concluded that there is a strong association with soil and conidial production and it was inferred that ‘the lesser the growth of dermatophyte on dissociated keratin in the ecology, the lesser is the chance of the dermatophyte likely to abundantly produce conidia’ [15]. Infections caused by geophiles can be described as saprobic–parasitic (S–P) infections, where the dermatophytes exist in saprobic phase in the soil in a near neutral pH and produce infectious propagules by utilizing the keratinous materials in the soil from decomposing structures and thus have the propensity to cause dermatophytosis in human and animals [16]. Such infections are rarely transmitted from man to man or lower animals to humans [17]. In the present case, even though the cook shared his accommodation with two other crew, none of them contracted dermatophytosis. A plethora of occupation-related dermatophytosis have been reported in the literature and a cook adds to the list of occupation as this attracts all the favourable factors for acquiring tinea, including profuse sweating and constant working in humid premises. Some notable infections transmitted by soil-borne propagules include M. gypseum, which is the most common geophilic pathogen causing tinea faciei following recreational exposure in soil [17]. Small epidemics and sporadic cases of tinea in cucumber growers due to occupational exposure in gardeners have been described [18]. Few rare case reports caused by geophilic species like Trichophyton terrestre, Trichophyton ajelloi and Paraphyton cookie (Microsporum cookie) have been reported, which were considered earlier as non-pathogenic to humans. In the present case, along with soil, the dog was also suspected as the source of N. gypsea because ‘burrowing and denning’ are the two characters of animals which predisposes them with infections by geophilic organisms [16, 17].
The clinical manifestations caused by various dermatophytes are indistinguishable and are not species specific [15]. Clinically, infections caused by N. gypsea (M. gypse um) were most commonly presented as tinea circinata on the glabrous skin, and tend to mount a highly inflammatory response and present either as pruritic scaly patches with pustules within the lesion or on the edges or as an eczematoid lesion [3, 19]. Several unique descriptions of lesions caused by N. gypsea in the immunocompetent hosts have been reported, which include concentric erythematous rings on the abdomen, a steroid modified tinea with squamous and inflammatory lesions with central hyperchromic zone and peripheral active zone filled with small vesicles, annular scaly erythematous lesions with pustules on the right forearm with involvement of villus hair, granulomatous and vesicular lesions with seborrheic dermatitis-like lesions on the lower legs, psoriasis-like lesions on the right earlobe and dystrophic right big toe onychomycosis etc. [3, 6]. Recently, an interesting case of Tinea incognito caused by N. gypsea was reported in a 22-year-old immunocompetent female from Madagascar with contact of cats and without any apparent contact with soil. The lesions were presented as solitary, round with circinate scaly plaques with well-defined erythematous lesions and peripheral active zone filled with small vesicles [19]. The present case also records a unique clinical presentation, which is in agreement with the previously described lesions caused by N. gypsea.
Antifungal susceptibility testing of dermatophytes is of significance while treating patients with recalcitrant dermatophytosis and those with tinea caused by non-anthropophilic dermatophytes and to guide the appropriate anti-fungal treatment. The geophilic dermatophytes are the most susceptible species attracting genetic variations [3]. Unlike the anthropophilic counterparts, which are the co-dominant or predominant pathogens in the current Indian scenario eg: T. rubrum and T. interdigitale with reported terbinafine resistance due to point mutations in squalene epoxidase gene, there are no reports of resistance in N. gypsea, this can be partly due to their relatively rare causative role in human infections [10, 11]. N. gypsea isolated from the present case showed increased MIC of griseofulvin, which is a drug considered in the treatment of tinea capitis, thus AFST data from this case also hints the clinicians to switch-over to alternative drugs like azoles and terbinafine when treating infections with geophilic dermatophytes causing tinea capitis. Topical therapy always holds an advantageous effect in the treatment of the glabrous skin tinea, especially during the initial days. Application of topical antifungal agents always produces high local concentrations of the drug, which enhances the reduction of the fungal load. Current scenario of recalcitrant and difficult to treat dermatophytosis in India warrants the use of a combination of topical and systemic antifungal agents [10]. Topical antifungal drugs such as butenafine cream given as monotherapy had shown a good clinical response in dermatophytosis of the glabrous skin caused by N. gypsea [20]. Our patient had complete resolution after 3-week treatment with systemic terbinafine 250 mg once daily and had no relapse for 1 year. He continues to be under follow-up. This good clinical response to terbinafine is uncommon in recent years, as there have been an increasing proportion of patients not responding to terbinafine and other commonly used regimens across India [10, 11]. Even though, 1 % luliconazole topical cream was recommended to the patient, upon follow-up history patient admitted to having discontinued the topical application after 5 days of application, probably due to the cost constraint. This supports the fact that application of topical luliconazole along with systemic terbinafine during the initial days reduced the fungal load and contributed to the alleviation of the severity of lesions.
In the current scenario of dermatophytosis in India, this case report stands as a unique example of a patient infected with N. gypsea showing complete clinical resolution after a short course of terbinafine. Epidemiology of dermatophytosis is noticing a crux change and a plethora of rare dermatophytes which have never been reported to cause infections are now implicated in causing dermatophytosis. Therefore, in this context, a close liaison between the clinician and the mycologist would help to confirm the aetiology and facilitate proper management. There is an absolute need for systematic studies for establishing the role of geophilic dermatophytes causing dermatomycosis and for this, all geographic locations where a geophile is suspected causing human infections, the immediate environment and soil should be studied for microflora and accordingly preventive measures should be laid down in a methodical way.
Funding information
The authors received no specific grant from any funding agency.
Acknowledgements
The authors wish to thank Dr Sri Shilpa Poojari, Assistant Professor and In-charge, Department of Dermatology (DVL), Dr Patnam Mahender Reddy Institute of Medical Sciences, Telangana, India for following-up the patient and Dr Vijay Raghavan, Director of Research, Saveetha University for co-supervising the methodology and framework of this mycology study. We are indebted to Professor Dr Arunaloke Chakrabarti and Mrs Sunita Gupta from NCCPF, Mycology Division, Department of Medical Microbiology, PGIMER, Chandigarh, India for accepting the request for sharing the information of the ITS sequence and antifungal susceptibility data. The authors also wish to acknowledge residents of Department of Dermatology and colleagues from Microbiology Department of Bhaskar Medical College and General Hospital, Telangana, India for their constant support in uplifting the mycology services with special consideration for dermatophytosis.
Authors contribution
All authors confirm accordance with the Committee on Publication Ethics (COPE) guidelines and have made a substantial contribution to the conception and design, acquisition of data, and/or analysis and interpretation of data. All of the authors have participated in the drafting of the article, revised it critically for important intellectual content and have read it before the approval of the final manuscript.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical statement
A written informed consent for publication was obtained from the patient. Humans in Research. All the procedures were in accordance with the ethical standards and approval of institutional human ethics committee which is responsible for human experimentation and were in strict compliance with the Helsinki Declaration of 1975, as revised in 1983.
Footnotes
Abbreviations: AFST, Antifungal susceptibility testing; AMO, amorolfine; CBS, Centraalbureau voor Schimmelcultures; CLSI, Clinical Laboratory Standards Institute; DTM, Dermatophytetest medium; GRI, griseofulvin; ITR, itraconazole; KOH, potassium hydroxide; M. gypseum, Microsporum gypseum; MIC, minimum inhibitory concentration; N. gypsea, Nannizzia gypsea; SER, sertaconazole; TER, terbinafine.
ITS Sequence GenBank Accession number MH715973.1 https://www.ncbi.nlm.nih.gov/nuccore/MH715973
References
- 1.de Hoog GS, Dukik K, Monod M, Packeu A, Stubbe D, et al. Toward a novel multilocus phylogenetic taxonomy for the dermatophytes. Mycopathologia. 2017;182:5–31. doi: 10.1007/s11046-016-0073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon MA, Perrin N. Little GN: differences in pathogencity between M. gypseum and M. fulvum . Sabouraudia. 1967;5:366–370. [PubMed] [Google Scholar]
- 3.Hayette MP, Sacheli R. Unusual species of dermatophytes: rarely identified or new? Mycopathologia. 2017;182:203–213. doi: 10.1007/s11046-016-0066-8. [DOI] [PubMed] [Google Scholar]
- 4.Singh I, Kushwaha RK. Dermatophytes and related keratinophilic fungi in soil of Parks and agricultural fields of Uttar Pradesh, India. Indian J Dermatol. 2010;55:306–308. doi: 10.4103/0019-5154.70700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chmel L, Buchvald J. Ecology and transmission of Microsporum gypseum from soil to man. Sabouraudia. 1970;8:149–156. doi: 10.1080/00362177085190791. [DOI] [PubMed] [Google Scholar]
- 6.Dolenc-Voljč M, Gasparič J. Human infections with Microsporum gypseum complex (Nannizzia gypsea) in Slovenia. Mycopathologia. 2017;182:1069–1075. doi: 10.1007/s11046-017-0194-9. [DOI] [PubMed] [Google Scholar]
- 7.Iyer SR, Bhargava R, Sharma M, Williamson D. Dermatophytic profile of Jaipur (Rajasthan) Anc Sci Life. 1995;14:181–186. [PMC free article] [PubMed] [Google Scholar]
- 8.Borman AM, Szekely A, Fraser M, Lovegrove S, Johnson EM, et al. A novel dermatophyte relative, Nannizzia perplicata sp. nov., isolated from a case of tinea corporis in the United Kingdom. Med Mycol. 2018 doi: 10.1093/mmy/myy099. [DOI] [PubMed] [Google Scholar]
- 9.Sharma R, Shouche Y. Nannizzia graeserae sp. nov., a new dermatophyte of geophilic clade isolated from vicinity of a barbershop in India. MSI (Kavaka) 2018;50:14–20. [Google Scholar]
- 10.Verma S, Madhu R. The great Indian epidemic of superficial dermatophytosis: an appraisal. Indian J Dermatol. 2017;62:227–236. doi: 10.4103/ijd.IJD_206_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudramurthy SM, Shankarnarayan SA, Dogra S, Shaw D, Mushtaq K, et al. Mutation in the squalene epoxidase gene of Trichophyton interdigitale and Trichophyton rubrum associated with allylamine resistance. Antimicrob Agents Chemother. 2018;62:e02522–17. doi: 10.1128/AAC.02522-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centraalbureau voor Schimmelcultures Dermatophytes its sequences database. http://www.westerdijkinstitute.nl/Dermatophytes/ 28 January 2019.
- 13.Kane J, Summerbell RC, Sigler L, Krajden S, Land G. In: Laboratory Handbook of Dermatophytes a Clinical Guide and Laboratory Handbook of Dermatophytes and Other Filamentous Fungi from Skin, Hair, and Nails. Kane J, editor. Belmont, Calif: Star Publishing Co; 1997. editor. [Google Scholar]
- 14.Rippon JW. In: Medical Mycology: The pathogenic Fungi and the Pathogenic Actinomycetes. 3rd ed. Rippon JW, editor. Philadelphia: Saunders; 1988. editor. [Google Scholar]
- 15.Weitzman I, Summerbell RC. The dermatophytes. Clin Microbiol Rev. 1995;8:240–259. doi: 10.1128/CMR.8.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ginter G. [Ecology, epidemiology and clinical symptomatology of Microsporum gypseum infections] Mycoses. 1989;32:531–535. [PubMed] [Google Scholar]
- 17.Mirhendi H, Makimura K, de Hoog GS, Rezaei-Matehkolaei A, Najafzadeh MJ, et al. Translation elongation factor 1-α gene as a potential taxonomic and identification marker in dermatophytes. Med Mycol. 2015;53:215–224. doi: 10.1093/mmy/myu088. [DOI] [PubMed] [Google Scholar]
- 18.De Vroey C. Ecological and epidemiological aspects in dermatophytoses. Zbl Bakt Hyg. 1984;257:234–239. doi: 10.1016/S0174-3031(84)80077-3. [DOI] [PubMed] [Google Scholar]
- 19.Soankasina AH, Rakotozandrindrainy N, Andrianteloasy S, Zafindraibe NJ, Rasamoelina T, et al. Dermatophyte infection caused by Nannizzia gypsea: a rare case report from Madagascar. Med Mycol Case Rep. 2018;20:7–9. doi: 10.1016/j.mmcr.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun PL, Ho HT. Concentric rings: an unusual presentation of tinea corporis caused by Microsporum gypseum . Mycoses. 2006;49:150–151. doi: 10.1111/j.1439-0507.2006.01204.x. [DOI] [PubMed] [Google Scholar]